Abstract
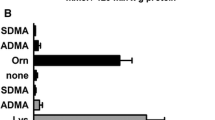
Among the members of the mitochondrial carrier family, there are transporters that catalyze the translocation of ornithine and related substrates, such as arginine, homoarginine, lysine, histidine, and citrulline, across the inner mitochondrial membrane. The mitochondrial carriers ORC1, ORC2, and SLC25A29 from Homo sapiens, BAC1 and BAC2 from Arabidopsis thaliana, and Ort1p from Saccharomyces cerevisiae have been biochemically characterized by transport assays in liposomes. All of them transport ornithine and amino acids with side chains terminating at least with one amine. There are, however, marked differences in their substrate specificities including their affinity for ornithine (KM values in the mM to μM range). These differences are most likely reflected by minor differences in the substrate binding sites of these carriers. The physiological role of the above-mentioned mitochondrial carriers is to link several metabolic pathways that take place partly in the cytosol and partly in the mitochondrial matrix and to provide basic amino acids for mitochondrial translation. In the liver, human ORC1 catalyzes the citrulline/ornithine exchange across the mitochondrial inner membrane, which is required for the urea cycle. Human ORC1, ORC2, and SLC25A29 are likely to be involved in the biosynthesis and transport of arginine, which can be used as a precursor for the synthesis of NO, agmatine, polyamines, creatine, glutamine, glutamate, and proline, as well as in the degradation of basic amino acids. BAC1 and BAC2 are implicated in some processes similar to those of their human counterparts and in nitrogen and amino acid metabolism linked to stress conditions and the development of plants. Ort1p is involved in the biosynthesis of arginine and polyamines in yeast.



Similar content being viewed by others
Abbreviations
- BAC1:
-
Basic amino acid carrier 1
- BAC2:
-
Basic amino acid carrier 2
- HHH:
-
Hyperornithinemia-hyperammonemia-homocitrullinuria
- MC:
-
Mitochondrial carrier
- NO:
-
Nitric oxide
- NOS:
-
NO synthase
- ORC1:
-
Ornithine carrier 1
- ORC2:
-
Ornithine carrier 2
- SLC25A29:
-
Member 29 of the SLC25 protein family
References
Agrimi G, Di Noia MA, Marobbio CMT, Fiermonte G, Lasorsa FM, Palmieri F (2004) Identification of the human mitochondrial S-adenosylmethionine transporter: bacterial expression, reconstitution, functional characterization and tissue distribution. Biochem J 379:183–190
Agrimi G, Russo A, Pierri CL, Palmieri F (2012) The peroxisomal NAD(+) carrier of Arabidopsis thaliana transports coenzyme A and its derivatives. J Bioenerg Biomembr 44:333–340
Al-Hassnan ZN, Rashed MS, Al-Dirbashi OY, Patay Z, Rahbeeni Z, Abu-Amero KK (2008) Hyperornithinemia-hyperammonemia-homocitrullinuria syndrome with stroke-like imaging presentation: clinical, biochemical and molecular analysis. J Neurol Sci 264:187–194
Alpoim PN, Sousa LP, Mota AP, Rios DR, Dusse LM (2015) Asymmetric Dimethylarginine (ADMA) in cardiovascular and renal disease. Clin Chim Acta 440:36–39
Arai Y, Hayashi M, Nishimura M (2008) Proteomic identification and characterization of a novel peroxisomal adenine nucleotide transporter supplying ATP for fatty acid beta-oxidation in soybean and Arabidopsis. Plant Cell 20:3227–3240
Bedhomme M, Hoffmann M, McCarthy EA, Gambonnet B, Moran RG, Rébeillé F, Ravanel S (2005) Folate metabolism in plants: an Arabidopsis homolog of the mammalian mitochondrial folate transporter mediates folate import into chloroplasts. J Biol Chem 280:34823–34831
Blemings KP, Crenshaw TD, Swick RW, Benevenga NJ (1994) Lysine-alpha-ketoglutarate reductase and saccharopine dehydrogenase are located only in the mitochondrial matrix in rat liver. J Nutr 124:1215–1221
Bouvier F, Linka N, Isner JC, Mutterer J, Weber APM, Camara B (2006) Arabidopsis SAMT1 defines a plastid transporter regulating plastid biogenesis and plant development. Plant Cell 18:3088–3105
Camacho J (2003) Cloning and characterization of human ORNT2: a second mitochondrial ornithine transporter that can rescue a defective ORNT1 in patients with the hyperornithinemia–hyperammonemia–homocitrullinuria syndrome, a urea cycle disorder. Mol Genet Metab 79:257–271
Camacho J, Rioseco-Camacho N (2009) The human and mouse SLC25A29 mitochondrial transporters rescue the deficient ornithine metabolism in fibroblasts of patients with the hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome. Pediatr Res 66:35–41
Camacho JA, Obie C, Biery B, Goodman BK, Hu CA, Almashanu S, Steel G, Casey R, Lambert M, Mitchell GA et al (1999) Hyperornithinaemia- syndrome is caused by mutations in a gene encoding a mitochondrial ornithine transporter. Nat Genet 22:151–158
Catoni E, Desimone M, Hilpert M, Wipf D, Kunze R, Schneider A, Flügge UI, Schumacher K, Frommer WB (2003) Expression pattern of a nuclear encoded mitochondrial arginine-ornithine translocator gene from Arabidopsis. BMC Plant Biol 3:1
Chappell JB, McGivan JD, Crompton M (1972) The molecular basis of biological transport. In: Woessner JFJ, Huijing F (eds) The molecular basis of biological transport. Academic Press, London, pp 55–81
Crabeel M, Soetens O, De Rijcke M, Pratiwi R, Pankiewicz R (1996) The ARG11 gene of Saccharomyces cerevisiae encodes a mitochondrial integral membrane protein required for arginine biosynthesis. J Biol Chem 271:25011–25018
Di Noia MA, Todisco S, Cirigliano A, Rinaldi T, Agrimi G, Iacobazzi V, Palmieri F (2014) The human SLC25A33 and SLC25A36 genes of solute carrier family 25 encode two mitochondrial pyrimidine nucleotide transporters. J Biol Chem 289:33137–33148
Ersoy Tunalı N, Marobbio CMT, Tiryakioğlu NO, Punzi G, Saygılı SK, Onal H, Palmieri F (2014) A novel mutation in the SLC25A15 gene in a Turkish patient with HHH syndrome: functional analysis of the mutant protein. Mol Genet Metab 112:25–29
Eubel H, Meyer EH, Taylor NL, Bussell JD, O’Toole N, Heazlewood JL, Castleden I, Small ID, Smith SM, Millar AH (2008) Novel proteins, putative membrane transporters, and an integrated metabolic network are revealed by quantitative proteomic analysis of Arabidopsis cell culture peroxisomes. Plant Physiol 148:1809–1829
Fiermonte G, Palmieri L, Todisco S, Agrimi G, Palmieri F, Walker JE (2002) Identification of the mitochondrial glutamate transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution of two human isoforms. J Biol Chem 277:19289–19294
Fiermonte G, Dolce V, David L, Santorelli FM, Dionisi-Vici C, Palmieri F, Walker JE (2003) The mitochondrial ornithine transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution of two human isoforms. J Biol Chem 278:32778–32783
Fukao Y, Hayashi Y, Mano S, Hayashi M, Nishimura M (2001) Developmental analysis of a putative ATP/ADP carrier protein localized on glyoxysomal membranes during the peroxisome transition in pumpkin cotyledons. Plant Cell Physiol 42:835–841
Galea E, Regunathan S, Eliopoulos V, Feinstein DL (1996) Reis DJ (1996) Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J 316:247–249
Ghafourifar P, Cadenas E (2005) Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26:190–195
Ghafourifar P, Asbury ML, Joshi SS, Kincaid ED (2005) Determination of mitochondrial nitric oxide synthase activity. Methods Enzymol 396:424–444
Hanfrey C, Sommer S, Mayer MJ, Burtin D, Michael AJ (2001) Arabidopsis polyamine biosynthesis: absence of ornithine decarboxylase and the mechanism of arginine decarboxylase activity. Plant J 27:551–560
He XJ, Mu RL, Cao WH, Zhang ZG, Zhang JS, Chen SY (2005) AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J 44:903–916
Herzfeld A, Mezl VA, Knox WE (1977) Enzymes metabolizing delta1-pyrroline-5-carboxylate in rat tissues. Biochem J 166:95–103
Hoyos ME, Palmieri L, Wertin T, Arrigoni R, Polacco JC, Palmieri F (2003) Identification of a mitochondrial transporter for basic amino acids in Arabidopsis thaliana by functional reconstitution into liposomes and complementation in yeast. Plant J 33:1027–1035
Illingworth C, Mayer MJ, Elliott K, Hanfrey C, Walton NJ, Michael AJ (2003) The diverse bacterial origins of the Arabidopsis polyamine biosynthetic pathway. FEBS Lett 549:26–30
Indiveri C, Krämer R, Palmieri F (1987) Reconstitution of the malate/aspartate shuttle from mitochondria. J Biol Chem 262:15979–15983
Indiveri C, Tonazzi A, Palmieri F (1992) Identification and purification of the ornithine/citrulline carrier from rat liver mitochondria. Eur J Biochem 207:449–454
Indiveri C, Palmieri L, Palmieri F (1994) Kinetic characterization of the reconstituted ornithine carrier from rat liver mitochondria. Biochim Biophys Acta 1188:293–301
Indiveri C, Tonazzi A, Stipani I, Palmieri F (1997) The purified and reconstituted ornithine/citrulline carrier from rat liver mitochondria: electrical nature and coupling of the exchange reaction with H + translocation. Biochem J 327:349–355
Indiveri C, Tonazzi A, Stipani I, Palmieri F (1999) The purified and reconstituted ornithine/citrulline carrier from rat liver mitochondria catalyses a second transport mode: orhithine +/H + exchange. Biochem J 711:705–711
Indiveri C, Tonazzi A, De Palma A, Palmieri F (2001) Kinetic mechanism of antiports catalyzed by reconstituted ornithine/citrulline carrier from rat liver mitochondria. Biochim Biophys Acta 1503:303–313
Kirchberger S, Tjaden J, Neuhaus HE (2008) Characterization of the Arabidopsis Brittle1 transport protein and impact of reduced activity on plant metabolism. Plant J 56:51–63
Kleber ME, Seppälä I, Pilz S, Hoffmann MM, Tomaschitz A, Oksala N, Raitoharju E, Lyytikäinen LP, Mäkelä KM, Laaksonen R et al (2013) Genome-wide association study identifies 3 genomic loci significantly associated with serum levels of homoarginine: the AtheroRemo Consortium. Circ Cardiovasc Genet 6:505–513
Kleinert H, Schwarz PM, Förstermann U (2003) Regulation of the expression of inducible nitric oxide synthase. Biol Chem 384:1343–1364
Kopyra M, Gwozdz EA (2003) Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol Biochem 41:1011–1017
Leroch M, Neuhaus HE, Kirchberger S, Zimmermann S, Melzer M, Gerhold J, Tjaden J (2008) Identification of a novel adenine nucleotide transporter in the endoplasmic reticulum of Arabidopsis. Plant Cell 20:438–451
Lim HK, Lim HK, Ryoo S, Benjo A, Shuleri K, Miriel V, Baraban E, Camara A, Soucy K, Nyhan D, Shoukas A, Berkowitz DE (2007) Mitochondrial arginase II constrains endothelial NOS-3 activity. Am J Physiol Heart Circ Physiol 293:H3317–H3324
Linka M, Weber AP (2005) Shuffling ammonia between mitochondria and plastids during photorespiration. Trends Plant Sci 10:461–465
Linka N, Theodoulou FL, Haslam RP, Linka M, Napier JA, Neuhaus HE, Weber APM (2008) Peroxisomal ATP import is essential for seedling development in Arabidopsis thaliana. Plant Cell 20:3241–3257
Litvinova L, Atochin DN, Fattakhov N, Vasilenko M, Zatolokin P, Kirienkova E (2015) Nitric oxide and mitochondria in metabolic syndrome. Front Physiol 6:20
Ludwig RA (1993) Arabidopsis chloroplasts dissimilate l-arginine and L-citrulline for use as N source. Plant Physiol 101:429–434
Mann GE, Yudilevich DL, Sobrevia L (2003) Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83:183–252
Marobbio CMT, Agrimi G, Lasorsa FM, Palmieri F (2003) Identification and functional reconstitution of yeast mitochondrial carrier for S-adenosylmethionine. EMBO J 22:5975–5982
Marobbio CMT, Punzi G, Pierri CL, Palmieri L, Calvello R, Panaro MA, Palmieri F (2015) Pathogenic potential of SLC25A15 mutations assessed by transport assays and complementation of Saccharomyces cerevisiae ORT1 null mutant. Mol Genet Metab 115:27–32
Martinelli D, Diodato D, Ponzi E, Monné M, Boenzi S, Bertini E, Fiermonte G, Dionisi-Vici C (2015) The hyperornithinemia–hyperammonemia-homocitrullinuria syndrome. Orphanet J Rare Dis 10:29
März W, Meinitzer A, Drechsler C, Pilz S, Krane V, Kleber ME, Fischer J, Winkelmann BR, Böhm BO, Ritz E et al (2010) Homoarginine, cardiovascular risk, and mortality. Circulation 122:967–975
McGuire DM, Gross MD, Elde RP, van Pilsum JF (1986) Localization of l-arginine-glycine amidinotransferase protein in rat tissues by immunofluorescence microscopy. J Histochem Cytochem 34:429–435
Mestichelli LJ, Gupta RN, Spenser ID (1979) The biosynthetic route from ornithine to proline. J Biol Chem 254:640–647
Miyamoto T, Kanazawa N, Kato S, Kawakami M, Inoue Y, Kuhara T, Inoue T, Takeshita K, Tsujino S (2001) Diagnosis of Japanese patients with HHH syndrome by molecular genetic analysis: a common mutation, R179X. J Human Genet 46:260–262
Miyamoto T, Kanazawa N, Hayakawa C, Tsujino S (2002) A novel mutation, P126R, in a Japanese patient with HHH syndrome. Pediatr Neurrol 26:65–67
Moali C, Boucher JL, Sari MA, Stuehr DJ, Mansuy D (1998) Substrate specificity of NO synthases: detailed comparison of l-arginine, homo-l-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-L-arginine. Biochemistry 37:10453–10460
Monné M, Palmieri F (2014) Antiporters of the mitochondrial carrier family. Curr Top Membr 73:289–320
Monné M, Miniero DV, Daddabbo L, Robinson AJ, Kunji ERS, Palmieri F (2012) Substrate specificity of the two mitochondrial ornithine carriers can be swapped by single mutation in substrate binding site. J Biol Chem 287:7925–7934
Monné M, Miniero DV, Iacobazzi V, Bisaccia F, Fiermonte G (2013a) The mitochondrial oxoglutarate carrier: from identification to mechanism. J Bioenerg Biomembr 45:1–13
Monné M, Palmieri F, Kunji ERS (2013b) The substrate specificity of mitochondrial carriers: mutagenesis revisited. Mol Membr Biol 30:149–159
Morris ML, Lee SC, Harper AE (1972) Influence of differential induction of histidine catabolic enzymes on histidine degradation in vivo. J Biol Chem 247:5793–5804
Morrissey J, McCracken R, Ishidoya S, Klahr S (1995) Partial cloning and characterization of an arginine decarboxylase in the kidney. Kidney Int 47:1458–1461
Nury H, Dahout-Gonzalez C, Trézéguet V, Lauquin G, Brandolin G, Pebay-Peyroula E (2005) Structural basis for lipid-mediated interactions between mitochondrial ADP/ATP carrier monomers. FEBS Lett 579:6031–6036
Palmieri F (1994) Mitochondrial carrier proteins. FEBS Lett 346:48–54
Palmieri F (2004) The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflügers Arch 447:689–709
Palmieri F (2008) Diseases caused by defects of mitochondrial carriers: a review. Biochim Biophys Acta 1777:564–578
Palmieri F (2013) The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med 34:465–484
Palmieri F (2014) Mitochondrial transporters of the SLC25 family and associated diseases: a review. J Inherit Metab Dis 37:565–575
Palmieri F, Pierri CL (2010a) Mitochondrial metabolite transport. Essays Biochem 47:37–52
Palmieri F, Pierri CL (2010b) Structure and function of mitochondrial carriers - role of the transmembrane helix P and G residues in the gating and transport mechanism. FEBS Lett 584:1931–1939
Palmieri L, De Marco V, Iacobazzi V, Palmieri F, Runswick MJ, Walker JE (1997) Identification of the yeast ARG-11 gene as a mitochondrial ornithine carrier involved in arginine biosynthesis. FEBS Lett 410:447–451
Palmieri L, Lasorsa FM, Vozza A, Agrimi G, Fiermonte G, Runswick MJ, Walker JE, Palmieri F (2000) Identification and functions of new transporters in yeast mitochondria. Biochim Biophys Acta 1459:363–369
Palmieri L, Pardo B, Lasorsa FM, del Arco A, Kobayashi K, Iijima M, Runswick MJ, Walker JE, Saheki T, Satrústegui et al (2001a) Citrin and aralar1 are Ca(2 +)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J 20:5060–5069
Palmieri L, Rottensteiner H, Girzalsky W, Scarcia P, Palmieri F, Erdmann R (2001b) Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J 20:5049–5059
Palmieri F, Agrimi G, Blanco E, Castegna A, Di Noia MA, Iacobazzi V, Lasorsa FM, Marobbio CMT, Palmieri L, Scarcia P et al (2006a) Identification of mitochondrial carriers in Saccharomyces cerevisiae by transport assay of reconstituted recombinant proteins. Biochim Biophys Acta 1757:1249–1262
Palmieri L, Todd CD, Arrigoni R, Hoyos ME, Santoro A, Polacco JC, Palmieri F (2006b) Arabidopsis mitochondria have two basic amino acid transporters with partially overlapping specificities and differential expression in seedling development. Biochim Biophys Acta 1757:1277–1283
Palmieri F, Rieder B, Ventrella A, Blanco E, Do PT, Nunes-Nesi A, Trauth AU, Fiermonte G, Tjaden J, Agrimi G et al (2009) Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD + carrier proteins. J Biol Chem 284:31249–31259
Palmieri F, Pierri CL, De Grassi A, Nunes-Nesi A, Fernie AR (2011) Evolution, structure and function of mitochondrial carriers: a review with new insights. Plant J 66:161–181
Papes F, Kemper EL, Cord-Neto G, Langone F, Arruda P (1999) Lysine degradation through the saccharopine pathway in mammals: involvement of both bifunctional and monofunctional lysine-degrading enzymes in mouse. Biochem J 344:555–563
Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJM, Brandolin G (2003) Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426:39–44
Planchais S, Cabassa C, Toka I, Justin AM, Renou JP, Savouré A, Carol P (2014) BASIC AMINO ACID CARRIER 2 gene expression modulates arginine and urea content and stress recovery in Arabidopsis leaves. Front Plant Sci 5:330
Porcelli V, Fiermonte G, Longo A, Palmieri F (2014) The human gene SLC25A29, of solute carrier family 25, encodes a mitochondrial transporter of basic amino acids. J Biol Chem 289:13374–13384
Ramachandra Reddy A, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Robinson AJ, Kunji ERS (2006) Mitochondrial carriers in the cytoplasmic state have a common substrate binding site. Proc Natl Acad Sci USA 103:2617–2622
Ruprecht JJ, Hellawell AM, Harding M, Crichton PG, McCoy AJ, Kunji ERS (2014) Structures of yeast mitochondrial ADP/ATP carriers support a domain-based alternating-access transport mechanism. Proc Natl Acad Sci USA 111:E426–E434
Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE (2008) Endothelial arginase II A novel target for the treatment of atherosclerosis. Circ Res 102:923–932
Salvi S, Dionisi-Vici C, Bertini E, Verardo M, Santorelli FM (2001) Seven novel mutations in the ORNT1 gene (SLC25A15) in patients with hyperornithinemia, hyperammonemia, and homocitrullinuria syndrome. Hum Mutat 18:460
Saraste M, Walker JE (1982) Internal sequence repeats and the path of polypeptide in mitochondrial ADP/ATP translocase. FEBS Lett 144:250–254
Satriano J, Matsufuji S, Murakami Y, Lortie MJ, Schwartz D, Kelly CJ, Hayashi S, Blantz RC (1998) Agmatine suppresses proliferation by frameshift induction of antizyme and attenuation of cellular polyamine levels. J Biol Chem 273:15313–15316
Sekoguchi E, Sato N, Yasui A, Fukada S, Nimura Y, Aburatani H, Ikeda K, Matsuura A (2003) A novel mitochondrial carnitine-acylcarnitine translocase induced by partial hepatectomy and fasting. J Biol Chem 278:38796–38802
Shargool PD, Jain JC, McKay G (1988) Ornithine biosynthesis, and arginine biosynthesis and degradation in plant cells. Phytochemistry 27:1571–1574
Shaul PW (2002) Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 64:749–774
Shih VE, Efron ML, Moser HW (1969) Hyperornithinemia, hyperammonemia, and homocitrullinuria. A new disorder of amino acid metabolism associated with myoclonic seizures and mental retardation. Am J Dis Child 117:83–92
Taira M, Valtersson U, Burkhardt B, Ludwig RA (2004) Arabidopsis thaliana GLN2-encoded glutamine synthetase is dual targeted to leaf mitochondria and chloroplasts. Plant Cell 16:2048–2058
Tessa A, Fiermonte G, Dionisi-Vici C, Paradies E, Baumgartner MR, Chien YH, Loguercio C, de Baulny HO, Nassogne MC, Schiff M et al (2009) Identification of novel mutations in the SLC25A15 gene in hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome: a clinical, molecular, and functional study. Hum Mutat 30:741–748
Thompson JF (1980) Arginine synthesis, proline synthesis, and related processes. In: Miflin BJ (ed) The Biochemistry of Plants, vol 5. Academic Press, New York, pp 375–403
Thuswaldner S, Lagerstedt JO, Rojas-Stütz M, Bouhidel K, Der C, Leborgne-Castel N, Mishra A, Marty F, Schoefs B, Adamska I et al (2007) Identification, expression, and functional analyses of a thylakoid ATP/ADP carrier from Arabidopsis. J Biol Chem 282:8848–8859
Todisco S, Di Noia MA, Castegna A, Lasorsa FM, Paradies E, Palmieri F (2014) The Saccharomyces cerevisiae gene YPR011c encodes a mitochondrial transporter of adenosine 5′-phosphosulfate and 3′-phospho-adenosine 5′-phosphosulfate. Biochim Biophys Acta 1837:326–334
Toka I, Planchais S, Cabassa C, Justin AM, De Vos D, Richard L, Savouré A, Carol P (2010) Mutations in the hyperosmotic stress-responsive mitochondrial BASIC AMINO ACID CARRIER 2 enhance proline accumulation in Arabidopsis. Plant Physiol 152:1851–1862
Tomaschitz A, Meinitzer A, Pilz S, Rus-Machan J, Genser B, Drechsler C, Grammer T, Krane V, Ritz E, Kleber ME et al (2014) Homoarginine, kidney function and cardiovascular mortality risk. Nephrol Dial Transplant 29:663–671
Tonazzi A, Giangregorio N, Palmieri F, Indiveri C (2005) Relationships of Cysteine and Lysine residues with the substrate binding site of the mitochondrial ornithine/citrulline carrier: an inhibition kinetic approach combined with the analysis of the homology structural model. Biochim Biophys Acta 1718:53–60
Tsujino S, Kanazawa N, Ohashi T, Eto Y, Saito T, Kira J, Yamada T (2000) Three novel mutations (G27E, insAAC, R179X) in the ORNT1 gene of Japanese patients with hyperornithinemia, hyperammonemia, and homocitrullinuria syndrome. Ann Neurol 47:625–631
Valle D, Simell O (2001) The hyperornithinemias. In: Beaudet AL, Sly WS, Valle D (eds) Scriver CR. The metabolic and molecular basis of inherited disease, New York, pp 1909–1964
Venekamp JH, Lampe JEM, Koot JTM (1989) Organic acids as sources or drought-induced proline synthesis in field bean plants, Vicia faba L. J Plant Physiol 133:654–659
Verma DPS, Zhang CS (1999) Regulation of proline and arginine biosynthesis in plants. In: Singh BK (ed) Plant amino acids: Biochemistry and biotechnology. Marcel Dekker, New York, pp 249–265
Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, Marmo R, Calcagnile VM, Palmieri L, Ricquier D et al (2014) UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA 111:960–965
Wang Y, Golledge J (2013) Neuronal nitric oxide synthase and sympathetic nerve activity in neurovascular and metabolic systems. Curr Neurovasc Res 10:81–89
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2013) Amino acids: Biochemistry and Nutrition. CRC Press, Taylor and Francis Group
Wu G, Morris SM (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Acknowledgments
This work was supported by grants from the Ministero dell’Università e della Ricerca (MIUR), the Comitato Telethon Fondazione Onlus n. GGP11139 and the Italian Human ProteomeNet no. RBRN07BMCT_009 (MIUR).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article is a review summarizing the results and conclusions of available publications that include previously performed studies on human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monné, M., Miniero, D.V., Daddabbo, L. et al. Mitochondrial transporters for ornithine and related amino acids: a review. Amino Acids 47, 1763–1777 (2015). https://doi.org/10.1007/s00726-015-1990-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-1990-5