Abstract
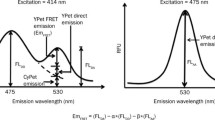
Protein–nucleic acid interaction is an important process in many biological phenomena. In this study, a fluorescence resonance energy transfer (FRET)-based protein–DNA binding assay has been developed, in which a fluorescent amino acid is genetically incorporated into a DNA-binding protein. A coumarin-containing amino acid was incorporated into a DNA-binding protein, and the mutant protein specifically produced a FRET signal upon binding to its cognate DNA labeled with a fluorophore. The protein–DNA binding affinity was then measured under equilibrium conditions. This method is advantageous for studying protein-nucleic acid interactions, because it is performed under equilibrium conditions, technically easy, and applicable to any nucleic acid-binding protein.




Similar content being viewed by others
References
Anderson BJ, Larkin C, Guja K, Schildbach JF (2008) Using fluorophore-labeled oligonucleotides to measure affinities of protein–DNA interactions. Methods Enzymol 450:253–272
Berg O, von Hippel P (1988) Selection of DNA binding sites by regulatory proteins. II. The binding specificity of cyclic AMP receptor protein to recognition sites. J Mol Biol 200:709–723
Boger DL, Desharnais J, Capps K (2003) Solution-phase combinatorial libraries: modulating cellular signaling by targeting protein–protein or protein–DNA interactions. Angew Chem Int Ed Engl 42:4138–4176
Brenowitz M, Senear DF, Shea MA, Ackers GK (1986) Quantitative DNase footprint titration: a method for studying protein–DNA interactions. Methods Enzymol 130:132–181
Cai Y-H, Huang H (2012) Advances in the study of protein–DNA interaction. Amino Acids 43:1141–1146
Chatterjee A, Guo J, Lee HS, Schultz PG (2013) A genetically encoded fluorescent probe in mammalian cells. J Am Chem Soc 135:12540–12543
De Crombrugghe B, Busby S, Buc H (1984) Cyclic AMP receptor protein: role in transcription activation. Science 224:831–838
Fried MG, Bromberg JL (1997) Factors that affect the stability of protein–DNA complexes during gel electrophoresis. Electrophoresis 18:6–11
Fried MG, Liu G (1994) Molecular sequestration stabilizes CAP-DNA complexes during polyacrylamide gel electrophoresis. Nucleic Acids Res 22:5054–5059
Hellman LM, Fried MG (2007) Electrophoretic mobility shift assay (EMSA) for detecting protein–nucleic acid interactions. Nat Protoc 2:1849–1861
Lee HS, Schultz PG (2008) Biosynthesis of a site-specific DNA cleaving protein. J Am Chem Soc 130:13194–13195
Lee HS, Guo J, Lemke EA, Dimla RD, Schultz PG (2009a) Genetic incorporation of a small, environmentally sensitive, fluorescent probe into proteins in Saccharomyces cerevisia. J Am Chem Soc 131:12921–12923
Lee HS, Dimla RD, Schultz PG (2009b) Protein–DNA photo-crosslinking with a genetically encoded benzophenone-containing amino acid. Bioorg Med Chem Lett 19:5222–5224
Liu CC, Schultz PG (2010) Adding new chemistries to the genetic code. Annu Rev Biochem 79:413–444
Oda M, Nakamura H (2000) Thermodynamic and kinetic analyses for understanding sequence-specific DNA recognition. Genes Cells 5:319–326
Oehler S, Alex R, Barker A (1999) Is nitrocellulose filter binding really a universal assay for protein–DNA interactions? Anal Biochem 268:330–336
Park N, Ryu J, Jang S, Lee HS (2012) Metal ion affinity purification of proteins by genetically incorporating metal-chelating amino acids. Tetrahedron 68:4649–4654
Parkinson G, Wilson C, Gunaseker A, Ebright YW, Ebright RH (1996) Structure of the CAP-DNA complex at 2.5 Å resolution: a complete picture of the protein–DNA interface. J Mol Biol 260:395–408
Pendergrast PS, Ebright YW, Ebright RH (1994) High-specificity DNA cleavage agent: design and application to kilobase and megabase DNA substrates. Science 265:959–962
Schultz SC, Shields GC, Steitz TA (1991) Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science 253:1001–1007
Speight LC, Muthusamy AK et al (2013) Efficient synthesis and in vivo incorporation of acridon-2-ylalanine, a fluorescent amino acid for lifetime and forster resonance energy transfer/luminescence resonance energy transfer studies. J Am Chem Soc 135:18806–18814
Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG (2006) A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA 103:9785–9789
Vossen KM, Fried MG (1997) Sequestration stabilizes lac repressor-DNA complexes during gel electrophoresis. Anal Biochem 245:85–92
Wang L, Schultz PG (2005) Expanding the genetic code. Angew Chem Int Ed Engl 44:34–66
Wang J, Xie J, Schultz PG (2006) A genetically encoded fluorescent amino acid. J Am Chem Soc 128:8738–8739
Woodbury CP, von Hippel PH (1983) On the determination of deoxyribonucleic acid-protein interactions parameters using the nitrocellulose filter-binding assay. Biochemistry 22:4730–4737
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2014003870), and the Sogang University Research Grant (201010048.01). We would like to thank P. G. Schultz for providing us the plasmids.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: D. Tsikas.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, H., Kang, H., Ko, W. et al. FRET-based analysis of protein-nucleic acid interactions by genetically incorporating a fluorescent amino acid. Amino Acids 47, 729–734 (2015). https://doi.org/10.1007/s00726-014-1900-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1900-2