Abstract
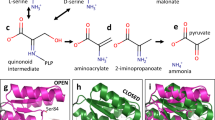
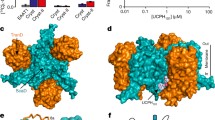
d-Serine is a non-proteinogenic amino acid that acts as a co-agonist of the NMDA receptors in the central nervous system. d-Serine is produced by human serine racemase (hSR), a homodimeric pyridoxal 5′-phosphate (PLP)-dependent enzyme that also catalyzes the physiologically relevant β-elimination of both l- and d-serine to pyruvate and ammonia. After improving the protein purification yield and stability, which had so far limited the biochemical characterization of hSR, we found that the catalytic activity is affected by halides, in the order fluoride > chloride > bromide. On the contrary, iodide elicited a complete inhibition, accompanied by a modulation of the tautomeric equilibrium of the internal aldimine. We also investigated the reciprocal effects of ATP and malonate, an inhibitor that reversibly binds at the active site, 20 Å away from the ATP-binding site. ATP increased ninefold the affinity of hSR for malonate and malonate increased 100-fold that of ATP, confirming an allosteric interaction between the two binding sites. To further investigate this allosteric communication, we probed the active site accessibility by quenching of the coenzyme fluorescence in the absence and presence of ATP. We found that ATP stabilizes a closed conformation of the external aldimine Schiff base, suggesting a possible mechanism for ATP-induced hSR activation.






Similar content being viewed by others
References
Ainscow EK, Mirshamsi S, Tang T, Ashford ML, Rutter GA (2002) Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K(+) channels. J Physiol 544:429–445. doi:10.1113/jphysiol.2002.022434
Amadasi A et al (2007) Pyridoxal 5′-phosphate enzymes as targets for therapeutic agents. Curr Med Chem 14:1291–1324. doi:10.2174/092986707780597899
Amori L, Katkevica S, Bruno A, Campanini B, Felici P, Mozzarelli A, Costantino G (2012) Design and synthesis of trans-2-substituted-cyclopropane-1-carboxylic acids as the first non-natural small molecule inhibitors of I-acetylserine sulfhydrylase. Med Chem Comm 3:1111–1116. doi:10.1039/c2md20100c
Balan L et al (2009) Feedback inactivation of d-serine synthesis by NMDA receptor-elicited translocation of serine racemase to the membrane. Proc Natl Acad Sci USA 106:7589–7594. doi:10.1073/pnas.0809442106
Benci S, Vaccari S, Mozzarelli A, Cook PF (1999) Time-resolved fluorescence of O-acetylserine sulfhydrylase. Biochim Biophys Acta 1429:317–330. doi:10.1016/S0167-4838(98)00229-5
Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT (2012) Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol 32:613–624. doi:10.1007/s10571-012-9808-4
Bertoldi M, Cellini B, Clausen T, Voltattorni CB (2002) Spectroscopic and kinetic analyses reveal the pyridoxal 5′-phosphate binding mode and the catalytic features of Treponema denticola cystalysin. Biochemistry 41(29):9153–9164. doi:10.1021/bi025649q
Bettati S et al (2000) Role of pyridoxal 5′-phosphate in the structural stabilization of O-acetylserine sulfhydrylase. J Biol Chem 275:40244–40251. doi:10.1074/jbc.M007015200
Bruno S, Schiaretti F, Burkhard P, Kraus JP, Janosik M, Mozzarelli A (2001) Functional properties of the active core of human cystathionine beta-synthase crystals. J Biol Chem 276:16–19. doi:10.1074/jbc.C000588200
Burkhard P, Tai CH, Jansonius JN, Cook PF (2000) Identification of an allosteric anion-binding site on O-acetylserine sulfhydrylase: structure of the enzyme with chloride bound. J Mol Biol 303:279–286. doi:10.1006/jmbi.2000.4109
Burridge N, Churchich JE (1974) Effects of potassium iodide on aspartate aminotransferase. Eur J Biochem 41:533–538. doi:10.1111/j.1432-1033.1974.tb03294.x
Campanini B et al (2003) Surface-exposed tryptophan residues are essential for O-acetylserine sulfhydrylase structure, function, and stability. J Biol Chem 278:37511–37519. doi:10.1074/jbc.M305138200
Campanini B et al (2005) Interaction of serine acetyltransferase with O-acetylserine sulfhydrylase active site: evidence from fluorescence spectroscopy. Prot Sci 14:2115–2124. doi:10.1110/ps.051492805
Campanini B, Spyrakis F, Peracchi A, Mozzarelli A (2013) Serine racemase: a key player in neuron activity and in neuropathologies. Front Biosci (Landmark Ed) 18:1112–1128. doi:10.2741/4167
Canu N, Ciotti MT, Pollegioni L (2014) Serine racemase: a key player in apoptosis and necrosis. Front Synaptic Neurosci 6:9. doi:10.3389/fnsyn.2014.00009
Chattopadhyay A et al (2007) Structure, mechanism, and conformational dynamics of O-acetylserine sulfhydrylase from Salmonella typhimurium: comparison of A and B isozymes. Biochemistry 46:8315–8330. doi:10.1021/bi602603c
Conti P, Tamborini L, Pinto A, Blondel A, Minoprio P, Mozzarelli A, De Micheli C (2011) Drug discovery targeting amino acid racemases. Chem Rev 111:6919–6946. doi:10.1021/cr2000702
De Miranda J, Panizzutti R, Foltyn VN, Wolosker H (2002) Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-d-aspartate (NMDA) receptor coagonist d-serine. Proc Natl Acad Sci USA 99:14542–14547. doi:10.1073/pnas.222421299
Ding X, Ma N, Nagahama M, Yamada K, Semba R (2011) Localization of d-serine and serine racemase in neurons and neuroglias in mouse brain. Neurol Sci 32:263–267. doi:10.1007/s10072-010-0422-2
Dixon SM, Li P, Liu R, Wolosker H, Lam KS, Kurth MJ, Toney MD (2006) Slow-binding human serine racemase inhibitors from high-throughput screening of combinatorial libraries. J Med Chem 49:2388–2397. doi:10.1021/jm050701c
Dominici P, Moore PS, Castellani S, Bertoldi M, Voltattorni CB (1997) Mutation of cysteine 111 in Dopa decarboxylase leads to active site perturbation. Protein Sci 6:2007–2015. doi:10.1002/pro.5560060921
Dumin E, Bendikov I, Foltyn VN, Misumi Y, Ikehara Y, Kartvelishvily E, Wolosker H (2006) Modulation of d-serine levels via ubiquitin-dependent proteasomal degradation of serine racemase. J Biol Chem 281:20291–20302. doi:10.1074/jbc.M601971200
Eftink MR, Ghiron CA (1981) Fluorescence quenching studies with proteins. Anal Biochem 114:199–227. doi:10.1016/0003-2697(81)90474-7
Faeder EJ, Hammes GG (1971) Kinetic studies of tryptophan synthetase. Interaction of l-serine, indole, and tryptophan with the native enzyme. Biochemistry 10:1041–1045. doi:10.1021/bi00782a016
Foltyn VN et al (2005) Serine racemase modulates intracellular d-serine levels through an alpha, beta-elimination activity. J Biol Chem 280:1754–1763. doi:10.1074/jbc.M405726200
Foltyn VN, Zehl M, Dikopoltsev E, Jensen ON, Wolosker H (2010) Phosphorylation of mouse serine racemase regulates d-serine synthesis. FEBS Lett 584:2937–2941. doi:10.1016/j.febslet.2010.05.022
Fujii K et al (2006) Serine racemase binds to PICK1: potential relevance to schizophrenia. Mol Psychiatry 11:150–157. doi:10.1038/sj.mp.4001776
Goto M et al (2009) Crystal structure of a homolog of mammalian serine racemase from Schizosaccharomyces pombe. J Biol Chem 284:25944–25952. doi:10.1074/jbc.M109.010470
Gut H, Pennacchietti E, John RA, Bossa F, Capitani G, De Biase D, Grütter MG (2006) Escherichia coli acid resistance: pH-sensing, activation by chloride and autoinhibition in GadB. EMBO J 25:2643–2651. doi:10.1038/sj.emboj.7601107
Hoffman HE, Jiraskova J, Ingr M, Zvelebil M, Konvalinka J (2009) Recombinant human serine racemase: enzymologic characterization and comparison with its mouse ortholog. Protein Express Purif 63:62–67. doi:10.1016/j.pep.2008.09.003
Inoue R et al (2014) Localization of serine racemase and its role in the skin. J Invest Dermatol. doi:10.1038/jid.2014.22
Ito T, Maekawa M, Hayashi S, Goto M, Hemmi H, Yoshimura T (2013) Catalytic mechanism of serine racemase from Dictyostelium discoideum. Amino Acids 44:1073–1084. doi:10.1007/s00726-012-1442-4
Jiraskova-Vanickova J, Ettrich R, Vorlova B, Hoffman HE, Lepsik M, Jansa P, Konvalinka J (2011) Inhibition of human serine racemase, an emerging target for medicinal chemistry. Curr Drug Targets 12:1037–1055. doi:10.2174/138945011795677755
Kaiser JT, Bruno S, Clausen T, Huber R, Schiaretti F, Mozzarelli A, Kessler D (2003) Snapshots of the cystine lyase C-DES during catalysis. Studies in solution and in the crystalline state. J Biol Chem 278:357–365. doi:10.1074/jbc.M209862200
Kallen RG et al (1985) Chemical and spectroscopic properties of pyridoxal and pyridoxamine phosphate. Metzler PCaDE Transaminases. John Wiley and Sons, New York, pp 37–108
Kim PM et al (2005) Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci USA 102:2105–2110. doi:10.1073/pnas.0409723102
Kossekova G, Miteva M, Atanasov B (1996) Characterization of pyridoxal phosphate as an optical label for measuring electrostatic potentials in proteins. J Photochem Photobiol B 32:71–79. doi:10.1016/1011-1344(95)07211-X
Labrie V, Wong AH, Roder JC (2012) Contributions of the d-serine pathway to schizophrenia. Neuropharmacol 62:1484–1503. doi:10.1016/j.neuropharm.2011.01.030
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Plenum Press, New York
Lipton SA (2006) Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov 5:160–170. doi:10.1038/nrd1958
Lodha PH, Hopwood EM, Manders AL, Aitken SM (2010) Residue N84 of yeast cystathionine beta-synthase is a determinant of reaction specificity. Biochim Biophys Acta 1804:1424–1431. doi:10.1016/j.bbapap.2010.02.010
Ma TM et al (2013) Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via d-serine depletion. Mol Psychiatry 18:557–567. doi:10.1038/mp.2012.97
Marabotti A, De Biase D, Tramonti A, Bettati S, Mozzarelli A (2001) Allosteric communication of tryptophan synthase. Functional and regulatory properties of the beta S178P mutant. J Biol Chem 276:17747–17753. doi:10.1074/jbc.M011781200
Marchetti M, Bruno S, Campanini B, Peracchi A, Mai N, Mozzarelli A (2013) ATP binding to human serine racemase is cooperative and modulated by glycine. FEBS J 280:5853–5863. doi:10.1111/febs.12510
Martinez del Pozo A, Yoshimura T, Bhatia MB, Futaki S, Manning JM, Ringe D, Soda K (1992) Inactivation of dimeric d-amino acid transaminase by a normal substrate through formation of an unproductive coenzyme adduct in one subunit. Biochemistry 31:6018–6023. doi:10.1021/bi00141a009
Maruyama K (1990) Activation of Pseudomonas ochraceae 4-hydroxy-4-methyl-2-oxoglutarate aldolase by inorganic phosphate. J Biochem 108:334–340
McClure GD Jr, Cook PF (1994) Product binding to the alpha-carboxyl subsite results in a conformational change at the active site of O-acetylserine sulfhydrylase-A: evidence from fluorescence spectroscopy. Biochemistry 33:1674–1683. doi:10.1021/bi00173a009
Molla G, Sacchi S, Bernasconi M, Pilone MS, Fukui K, Polegioni L (2006) Characterization of human d-amino acid oxidase. FEBS Lett 580:2358–2364. doi:10.1016/j.febslet.2006.03.045
Mozzarelli A, Bettati S (2006) Exploring the pyridoxal 5′-phosphate-dependent enzymes. Chem Rec 6:275–287. doi:10.1002/tcr.20094
Mozzarelli A et al (2011) The multifaceted pyridoxal 5′-phosphate-dependent O-acetylserine sulfhydrylase. Biochim Biophys Acta 1814:1497–1510. doi:10.1016/j.bbapap.2011.04.011
Mustafa AK et al (2007) Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of d-serine formation. Proc Natl Acad Sci USA 104:2950–2955. doi:10.1073/pnas.0611620104
Mustafa AK et al (2010) Serine racemase deletion protects against cerebral ischemia and excitotoxicity. J Neurosci 30:1413–1416. doi:10.1523/JNEUROSCI.4297-09.2010
Nagayoshi C, Ishibashi M, Kita Y, Matsuoka M, Nishimoto I, Tokunaga M (2005) Expression, refolding and characterization of human brain serine racemase in Escherichia coli with N-terminal His-tag. Protein Pept Lett 12:487–490. doi:10.2174/0929866054395284
Nagayoshi C, Ishibashi M, Tokunaga M (2009) Purification and characterization of human brain serine racemase expressed in moderately halophilic bacteria. Protein Pept Lett 16:201–206. doi:10.2174/092986609787316261
Neidle A, Dunlop DS (2002) Allosteric regulation of mouse brain serine racemase. Neurochem Res 27:1719–1724. doi:10.1023/A:1021607715824
Peracchi A, Mozzarelli A, Rossi GL (1995) Monovalent cations affect dynamic and functional-properties of the tryptophan synthase alpha(2)beta(2) complex. Biochemistry 34:9459–9465. doi:10.1021/Bi00029a022
Perutz MF, Shih DT, Williamson D (1994) The chloride effect in human haemoglobin. A new kind of allosteric mechanism. J Mol Biol 239:555–560. doi:10.1006/jmbi.1994.1394
Phillips RS, Demidkina TV, Zakomirdina LN, Bruno S, Ronda L, Mozzarelli A (2002) Crystals of tryptophan indole-lyase and tyrosine phenol-lyase form stable quinonoid complexes. J Biol Chem 277:21592–21597. doi:10.1074/jbc.M200216200
Quazi F, Aitken SM (2009) Characterization of the S289A, D mutants of yeast cystathionine beta-synthase. Biochim Biophys Acta 1794:892–897. doi:10.1016/j.bbapap.2009.02.007
Raboni S, Bettati S, Mozzarelli A (2009) Tryptophan synthase: a mine for enzymologists. Cell Mol Life Sci 66:2391–2403. doi:10.1007/s00018-009-0028-0
Rust E, Martin DL, Chen CH (2001) Cofactor and tryptophan accessibility and unfolding of brain glutamate decarboxylase. Arch Biochem Biophys 392:333–340. doi:10.1006/abbi.2001.2466
Sacchi S, Caldinelli L, Cappelletti P, Pollegioni L, Molla G (2012) Structure-function relationships in human d-amino acid oxidase. Amino Acids 43:1833–1850. doi:10.1007/s00726-012-1345-4
Salsi E et al (2010) Design of O-acetylserine sulfhydrylase inhibitors by mimicking nature. J Med Chem 53:345–356. doi:10.1021/jm901325e
Schiaretti F, Bettati S, Viappiani C, Mozzarelli A (2004) pH dependence of tryptophan synthase catalytic mechanism: I. The first stage, the beta-elimination reaction. J Biol Chem 279:29572–29582. doi:10.1074/jbc.M401895200
Schnackerz KD, Tai CH, Simmons JW 3rd, Jacobson TM, Rao GS, Cook PF (1995) Identification and spectral characterization of the external aldimine of the O-acetylserine sulfhydrylase reaction. Biochemistry 34:12152–12160
Silver IA, Erecinska M (1997) Energetic demands of the Na+/K+ ATPase in mammalian astrocytes. Glia 21:35–45 10.1002
Smith MA et al (2010) The structure of mammalian serine racemase: evidence for conformational changes upon inhibitor binding. J Biol Chem 285:12873–12881. doi:10.1074/jbc.M109.050062
Spyrakis F et al (2013) Fine tuning of the active site modulates specificity in the interaction of O-acetylserine sulfhydrylase isozymes with serine acetyltransferase. Biochim Biophys Acta 1834:169–181. doi:10.1016/j.bbapap.2012.09.009
Spyrakis F et al (2014) Targeting cystalysin, a virulence factor of Treponema denticola-supported periodontitis. Chem Med Chem. doi:10.1002/cmdc.201300527
Strambini GB, Cioni P, Peracchi A, Mozzarelli A (1992) Conformational-changes and subunit communication in tryptophan synthase—effect of substrates and substrate-analogs. Biochemistry 31:7535–7542. doi:10.1021/Bi00148a014
Strambini GB, Cioni P, Cook PF (1996) Tryptophan luminescence as a probe of enzyme conformation along the O-acetylserine sulfhydrylase reaction pathway. Biochemistry 35:8392–8400. doi:10.1021/bi952919e
Strisovsky K, Jiraskova J, Mikulova A, Rulisek L, Konvalinka J (2005) Dual substrate and reaction specificity in mouse serine racemase: identification of high-affinity dicarboxylate substrate and inhibitors and analysis of the beta-eliminase activity. Biochemistry 44:13091–13100. doi:10.1021/bi051201o
Tian H et al (2010) Identification of the structural determinants for the stability of substrate and aminoacrylate external Schiff bases in O-acetylserine sulfhydrylase-A. Biochemistry 49:6093–6103. doi:10.1021/bi100473v
Toney MD (2011) Pyridoxal phosphate enzymology. Biochim Biophys Acta 1814:1405–1406. doi:10.1016/j.bbapap.2011.08.007
Traynelis SF et al (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62:405–496. doi:10.1124/pr.109.002451
Wang W, Barger SW (2011) Roles of quaternary structure and cysteine residues in the activity of human serine racemase. BMC Biochem 12:63. doi:10.1186/1471-2091-12-63
Wild JR, Belser WL, O’Donovan GA (1976) Unique aspects of the regulation of the aspartate transcarbamylase of Serratia marcescens. J Bacteriol 128:766–775
Wolosker H, Blackshaw S, Snyder SH (1999) Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci USA 96:13409–13414. doi:10.1073/pnas.96.23.13409
Zhang J, Cheltsov AV, Ferreira GC (2005) Conversion of 5-aminolevulinate synthase into a more active enzyme by linking the two subunits: spectroscopic and kinetic properties. Protein Sci 14:1190–1200. doi:10.1110/ps.041258305
Zhuang Z, Yang B, Theus MH, Sick JT, Bethea JR, Sick TJ, Liebl DJ (2010) EphrinBs regulate d-serine synthesis and release in astrocytes. J Neurosci 30:16015–16024. doi:10.1523/JNEUROSCI.0481-10.2010
Conflict of interest
No conflicts of interest declared by any of the authors.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marchetti, M., Bruno, S., Campanini, B. et al. Regulation of human serine racemase activity and dynamics by halides, ATP and malonate. Amino Acids 47, 163–173 (2015). https://doi.org/10.1007/s00726-014-1856-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1856-2