Abstract
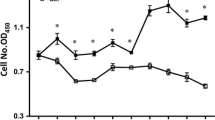
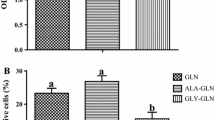
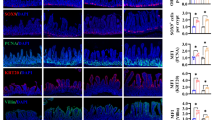
Neonates (including human infants) require l-glutamine (Gln) for optimal intestinal health. This study tested the hypothesis that Gln enhances enterocyte growth via both mammalian target of rapamycin (mTOR) and AMP-activated kinase (AMPK) signaling pathways. Intestinal porcine epithelial cells (IPEC-1) were cultured for 3 days in Gln-free Dulbecco’s modified Eagle medium containing 0 or 2 mM Gln. To determine the role of mTOR and AMPK on cell growth, additional experiments were conducted where medium contained 2 mM Gln and 10 nM rapamycin (Rap, an inhibitor of mTOR) or 1 μM compound C (an inhibitor of AMPK). IPEC-1 cell growth increased with increasing concentrations of Gln from 0 to 2 mM. Compared with 0 mM Gln, 2 mM Gln increased (P < 0.05) the amounts of phosphorylated 4E-binding protein 1 (4E-BP1) and ribosomal protein S6 kinase (p70S6 kinase) proteins but did not affect abundances of total or phosphorylated AMPK protein. Gln also increased mRNA levels for Bcl-2, mTOR, p70S6 kinase, 4E-BP1, COX7C, ASCT2, ODC, SGLT-1, CFTR, Na+/K+-ATPase, HSP70, and ZO-1. Similarly, cells cultured with Rap and Gln exhibited higher (P < 0.05) abundances of phosphorylated 4E-BP1 and p70S6 kinase proteins than the Rap-only group, whereas abundances of phosphorylated mTOR and 4E-BP1 proteins were increased when AMPK was inhibited by compound C. Conversely, the amount of phosphorylated AMPK increased when mTOR was inhibited by Rap, suggesting a negative cross-talk between mTOR and AMPK. Collectively, these results indicate that Gln stimulates enterocyte growth by activating the mTOR signaling pathway independently of AMPK.








Similar content being viewed by others
Abbreviations
- AMPK:
-
Adenosine 5′-monophosphate (AMP)-activated protein kinase
- ASCT2:
-
Na+-neutral AA exchanger 2
- Bcl-2:
-
B-cell lymphoma 2
- Cc:
-
Compound C
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- COX7C:
-
Cytochrome c oxidase subunit I
- HSP70:
-
Heat shock protein 70
- MAPK6:
-
Mitogen-activated protein kinase 6
- mTOR:
-
Mammalian target of rapamycin
- ODC:
-
Ornithine decarboxylase
- pBD-1:
-
Porcine β-defense 1
- Rap:
-
Rapamycin
- SGLT-1:
-
Sodium/glucose co-transporter-1
- Sirt 1:
-
Sirtuin 1
- 4E-BP1:
-
4E-binding protein-1
- ZO-1:
-
Zonula occludens-1
References
Ahmad S, White CW, Chang LY et al (2001) Glutamine protects mitochondrial structure and function in oxygen toxicity. Am J Physiol Lung Cell Mol Physiol 280:L779–L791
Ban K, Kozar RA (2010) Glutamine protects against apoptosis via downregulation of Sp3 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 299:G1344–G1353
Barnabé N, Butler M (2000) The effect of glucose and glutamine on the intracellular nucleotide pool and oxygen uptake rate of a murine hybridoma. Cytotechnology 34:47–57
Bauchart-Thevret C, Cui L, Wu G et al (2010) Arginine-induced stimulation of protein synthesis and survival in IPEC-J2 cells is mediated by mTOR but not nitric oxide. Am J Physiol Endocrinol Metab 299:E899–E909
Bruel T, Guibon R, Melo S et al. (2010) Epithelial induction of porcine suppressor of cytokine signaling 2 (SOCS2) gene expression in response to Entamoeba histolytica. Dev Comp Immunol 34:562–571
Bungard CI, McGIVAN JD (2004) Glutamine availability up-regulates expression of the amino acid transporter protein ASCT2 in HepG2 cells and stimulates the ASCT2 promoter. Biochem J 382:27–32
Fu WJ, Stromberg AJ, Viele K et al (2010) Statistics and bioinformatics in nutritional sciences: analysis of complex data in the era of systems biology. J Nutr Biochem 21:561–572
Fumarola C, LaMonica S, Guidotti G (2005) Amino acid signaling through the mammalian target of rapamycin (mTOR) pathway: role of glutamine and of cell shrinkage. J Cell Physiol 204:155–165
Gleason CE, Lu D, Witters LA et al (2007) The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem 282:10341–10351
Groening P, Huang Z, La Gamma EF et al (2011) Glutamine restores myocardial cytochrome C oxidase activity and improves cardiac function during experimental sepsis. JPEN J Parenter Enter Nutr 35:249–254
Haynes TE, Li P, Li XL et al (2009) l-Glutamine or l-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37:131–142
Hinshaw DB, Burger JM (1990a) Protective effect of glutamine on endothelial cell ATP in oxidant injury. J Surg Res 49:222–227
Hinshaw DB, Burger JM (1990b) Protective effect of glutamine on endothelial cell ATP in oxidant injury. J Surg Res 49:222–227
Hou Y, Wang L, Yi D et al (2013) N-acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids 45:513–522
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590
Jobgen WS, Fried SK, Fu WJ et al (2006) Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17:571–588
Kandil HM, Argenzio RA, Chen W et al (1995) l-Glutamine and l-asparagine stimulate ODC activity and proliferation in a porcine jejunal enterocyte line. Am J Physiol 269:G591–G599
Kim J, Song G, Wu G et al (2013) Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF-4EBP1 signal transduction pathway. Biol Reprod 88:113
Lambertucci AC, Lambertucci RH, Hirabara SM et al (2012) Glutamine supplementation stimulates protein-synthetic and inhibits protein-degradative signaling pathways in skeletal muscle of diabetic rats. PLoS ONE 7(12):e50390
Li P, Kim SW, Li X et al (2008) Dietary supplementation with cholesterol and docosahexaenoic acid increases the activity of the arginine-nitric oxide pathway in tissues of young pigs. Nitric Oxide 19:259–265
Li P, Knabe DA, Kim SW et al (2009) Lactating porcine mammary tissue catabolizes branched-chain amino acids for glutamine and aspartate synthesis. J Nutr 139:1502–1509
Li L, Chen Y, Gibson SB (2013) Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signal 25:50–65
Liu X, Yuan H, Niu Y et al (2012) The role of AMPK/mTOR/S6K1 signaling axis in mediating the physiological process of exercise-induced insulin sensitization in skeletal muscle of C57BL/6 mice. Biochim Biophys Acta 1822:1716–1726
Long YC, Cheng Z, Copps KD et al (2011) Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Mol Cell Biol 31:430–441
Mates JM, Perez-gomez C, Nunez de Castro I et al (2002) Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol 34:439–458
Meurens F, Berri M, Auray G et al (2009) Early immune response following Salmonella enterica subspecies enterica serovar Typhimurium infection in porcine jejunal gut loops. Vet Res 40:5
Motoshima H, Goldstein BJ, Igata M et al (2006) AMPK and cell proliferation-AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574:63–71
Mounier R, Lantier L, Leclerc J et al (2011) Antagonistic control of muscle cell size by AMPK and mTORC1. Cell Cycle 10:2640–2646
Nygard A, Jørgensen CB, Cirera S et al. (2007) Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol Biol 8:67
Reeds PJ, Burrin DG (2001) Glutamine and the bowel. J Nutr 131:2505S–2508S
Rezaei R, Knabe DA, Tekwe CD et al (2013a) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
Rezaei R, Wang WW, Wu ZL et al (2013b) Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotechnol 4:7
Rhoads JM, Wu G (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37:111–122
Rhoads JM, Argenzio RA, Chen W et al (1997) l-Glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol 272:G943–G953
Ropeleski MJ, Riehm J, Baer KA et al (2005) Anti-apoptotic effects of l-glutamine-mediated transcriptional modulation of the heat shock protein 72 during heat shock. Gastroenterology 129:170–184
Tan BE, Yin YL, Kong XF et al (2010) l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9:799–809
Wang J, Chen L, Li P et al (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138:1025–1032
Wang WW, Wu ZL, Dai ZL et al (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477
Wang B, Wu G, Zhou ZG et al (2014a) Glutamine and intestinal barrier function. Amino Acids. doi:10.1007/s00726-014-1773-4
Wang WW, Dai ZL, Wu ZL et al (2014b) Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 46:2037–2045
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2010) Functional amino acids in growth, reproduction, and health. Adv Nutr 1:31–37
Wu G (2013) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu G (2014) Dietary requirements of synthesizable amino acids by animals: A paradigm shift in protein nutrition. J Anim Sci Biotechnol 5:34
Wu G, Knabe DA, Yan W et al (1995) Glutamine and glucose metabolism in enterocytes of the neonatal pig. Am J Physiol Regulatory Integr Comp Physiol 268:R334–R342
Wu G, Meier SA, Knabe DA (1996) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 126:2578–2584
Wu G, Bazer FW, Johnson GA et al (2011) Important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu G, Wu ZL, Dai ZL et al (2013) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Wu G, Bazer FW, Dai ZL et al (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Xi P, Jiang Z, Dai Z et al (2012) Regulation of protein turnover by l-glutamine in porcine intestinal epithelial cells. J Nutr Biochem 23:1012–1017
Xu HD, Wu D, Gu JH et al (2013) The pro-survival role of autophagy depends on Bcl-2 under nutrition stress conditions. PLoS ONE 8:e63232
Yao K, Yin Y, Li X et al (2012) Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids 42:2491–2500
Yin YL, Yao K, Liu ZJ et al (2010) Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486
Yoo SS, Field CJ, McBurney MI (1997) Glutamine supplementation maintains intramuscular glutamine concentrations and normalizes lymphocyte function in infected early weaned pigs. J Nutr 127:2253–2259
Zhu Y, Lin G, Dai Z et al (2014) l-Glutamine deprivation induces autophagy and alters the mTOR and MAPK signaling pathways in porcine intestinal epithelial cells. Amino Acids. doi:10.1007/s00726-014-1785-0
Acknowledgments
This research was jointly supported by National Basic Research Program of China (No. 2012CB126305), National Natural Science Foundation of China (No. 31372319 and 31402084), Hubei Provincial Research and Development Program (No. 2010BB023), Natural Science Foundation of Hubei Province (No. 2013CFA097, 2013CFB325, 2012FFB04805, 2011CDA131), the Hubei Hundred Talent program, Agriculture and Food Research Initiative Competitive Grant (2014-67015-21770) of the USDA National Institute of Food and Agriculture, and Texas AgriLife Research (H-82000). All these funding agencies had no role in the design, analysis or writing of this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, D., Hou, Y., Wang, L. et al. l-Glutamine enhances enterocyte growth via activation of the mTOR signaling pathway independently of AMPK. Amino Acids 47, 65–78 (2015). https://doi.org/10.1007/s00726-014-1842-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1842-8