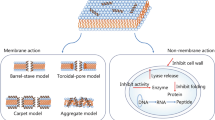
Abstract
The increasing resistance of pathogens to antibiotics causes a huge clinical burden that places great demands on academic researchers and the pharmaceutical industry for resolution. Antimicrobial peptides, part of native host defense, have emerged as novel potential antibiotic alternatives. Among the different classes of antimicrobial peptides, proline-rich antimicrobial peptides, predominantly sourced from insects, have been extensively investigated to study their specific modes of action. In this review, we focus on recent developments in these peptides. They show a variety of modes of actions, including mechanism shift at high concentration, non-lytic mechanisms, as well as possessing different intracellular targets and lipopolysaccharide binding activity. Furthermore, proline-rich antimicrobial peptides display the ability to not only modulate the immune system via cytokine activity or angiogenesis but also possess properties of penetrating cell membranes and crossing the blood brain barrier suggesting a role as potential novel carriers. Ongoing studies of these peptides will likely lead to the development of more potent antimicrobial peptides that may serve as important additions to the armoury of agents against bacterial infection and drug delivery.


Similar content being viewed by others
Abbreviations
- AMPs:
-
Antimicrobial peptides
- PrAMPs:
-
Proline-rich antimicrobial peptides
- LPS:
-
Lipopolysaccharide
- CPPs:
-
Cell penetrating peptides
- BBB:
-
Blood brain barrier
References
Agerberth B, Lee JY, Bergman T, Carlquist M, Boman HG, Mutt V, Jornvall H (1991) Amino acid sequence of PR-39. Isolation from pig intestine of a new member of the family of proline-arginine-rich antibacterial peptides. Eur J Biochem 202:849–854
Anbanandam A, Albarado DC, Tirziu DC, Simons M, Veeraraghavan S (2008) Molecular basis for proline- and arginine-rich peptide inhibition of proteasome. J Mol Biol 384:219–227
Avitabile C, D’Andrea LD, Romanelli A (2014) Circular dichroism studies on the interactions of antimicrobial peptides with bacterial cells. Sci Rep 4:4293
Bahar A, Ren D (2013) Antimicrobial peptides. Pharmaceuticals 6:1543–1575
Baumann T, Kämpfer U, Schürch S, Schaller J, Largiadèr C, Nentwig W, Kuhn-Nentwig L (2010) Ctenidins: antimicrobial glycine-rich peptides from the hemocytes of the spider Cupiennius salei. Cell Mol Life Sci 67:2787–2798
Bencivengo A-M, Cudic M, Hoffmann R, Otvos L Jr (2001) The efficacy of the antibacterial peptide, pyrrhocoricin, is finely regulated by its amino acid residues and active domains. Lett Pept Sci 8:201–209
Benincasa M, Pelillo C, Zorzet S, Garrovo C, Biffi S, Gennaro R, Scocchi M (2010) The proline-rich peptide Bac7(1–35) reduces mortality from Salmonella typhimurium in a mouse model of infection. BMC Microbiol 10:178
Berthold N, Hoffmann R (2014) Cellular uptake of apidaecin 1b and related analogs in Gram-negative bacteria reveals novel antibacterial mechanism for proline-rich antimicrobial peptides. Protein Pept Lett 21:391–398
Boman HG, Agerberth B, Boman A (1993) Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect Immun 61:2978–2984
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250
Brötz H, Bierbaum G, Leopold K, Reynolds PE, Sahl H-G (1998) The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob Agents Chemother 42:154–160
Bulet P, Dimarcq JL, Hetru C, Lagueux M, Charlet M, Hegy G, Van Dorsselaer A, Hoffmann JA (1993) A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution. J Biol Chem 268:14893–14897
Bulet P, Hetru C, Dimarcq JL, Hoffmann D (1999) Antimicrobial peptides in insects; structure and function. Dev Comp Immunol 23:329–344
Butler MS, Blaskovich MA, Cooper MA (2013) Antibiotics in the clinical pipeline in 2013. J Antibiot (Tokyo) 66:571–591
Cannon B (2014) Microbiology: resistance fighters. Nature 509:S6–S8
Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–395
Cassone M, Otvos L (2010) Synergy among antibacterial peptides and between peptides and small-molecule antibiotics. Expert Rev Anti Infect Ther 8:703–716
Cassone M, Frith N, Vogiatzi P, Wade JD, Otvos L (2009) Induced resistance to the designer proline-rich antimicrobial peptide A3-APO does not involve changes in the intracellular target DnaK. Int J Pept Res Ther 15:121–128
Casteels P, Ampe C, Jacobs F, Vaeck M, Tempst P (1989) Apidaecins: antibacterial peptides from honeybees. EMBO J 8:2387
Chan DI, Prenner EJ, Vogel HJ (2006) Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta 1758:1184–1202
Chen W, Luo L (2009) Classification of antimicrobial peptide using diversity measure with quadratic discriminant analysis. J Microbiol Methods 78:94–96
Chesnokova LS, Slepenkov SV, Witt SN (2004) The insect antimicrobial peptide, l-pyrrhocoricin, binds to and stimulates the ATPase activity of both wild-type and lidless DnaK. FEBS Lett 565:65–69
Chitnis SN, Prasad KN, Bhargava PM (1987) Bacteriolytic activity of seminalplasmin. J Gen Microbiol 133:1265–1271
Chitnis SN, Prasad KSN, Bhargava PM (1990) Isolation and characterization of autolysis-defective mutants of Escherichia coli that are resistant to the lytic activity of seminalplasmin. J Gen Microbiol 136:463–469
Cho JH, Park CB, Yoon YG, Kim SC (1998) Lumbricin I, a novel proline-rich antimicrobial peptide from the earthworm: purification, cDNA cloning and molecular characterization. Biochim Biophys Acta 1408:67–76
Cully M (2014) Public health: the politics of antibiotics. Nature 509:S16–S17
Dagan A, Efron L, Gaidukov L, Mor A, Ginsburg H (2002) In vitro antiplasmodium effects of dermaseptin S4 derivatives. Antimicrob Agents Chemother 46:1059–1066
de Souza Cândido E, e Silva Cardoso MH, Sousa DA, Viana JC, de Oliveira-Júnior NG, Miranda V, Franco OL (2014) The use of versatile plant antimicrobial peptides in agribusiness and human health. Peptides 55:65–78
Delgado MA, Rintoul MR, Farı́as RN, Salomón RA (2001) Escherichia coli RNA polymerase is the target of the cyclopeptide antibiotic microcin J25. J Bacteriol 183:4543–4550
Destoumieux D, Munoz M, Bulet P, Bachere E (2000) Penaeidins, a family of antimicrobial peptides from penaeid shrimp (Crustacea, Decapoda). Cell Mol Life Sci 57:1260–1271
Dimarcq J-L, Bulet P, Hetru C, Hoffmann J (1998) Cysteine-rich antimicrobial peptides in invertebrates. Pept Sci 47:465–477
Dmitriev RI, Ropiak HM, Yashunsky DV, Ponomarev GV, Zhdanov AV, Papkovsky DB (2010) Bactenecin 7 peptide fragment as a tool for intracellular delivery of a phosphorescent oxygen sensor. FEBS J 277:4651–4661
Dong N, Ma Q, Shan A, Lv Y, Hu W, Gu Y, Li Y (2012) Strand length-dependent antimicrobial activity and membrane-active mechanism of arginine- and valine-rich β-hairpin-like antimicrobial peptides. Antimicrob Agents Chemother 56:2994–3003
El-Andaloussi S, Järver P, Johansson HJ, Langel Ü (2007) Cargo-dependent cytotoxicity and delivery efficacy of cell-penetrating peptides: a comparative study. Biochem J 407:285–292
Fasano A (1998) Innovative strategies for the oral delivery of drugs and peptides. Trends Biotechnol 16:152–157
Fernandez DI, Gehman JD, Separovic F (2009) Membrane interactions of antimicrobial peptides from Australian frogs. Biochim Biophys Acta 1788:1630–1638
Fernández-Vidal M, Jayasinghe S, Ladokhin AS, White SH (2007) Folding amphipathic helices into membranes: amphiphilicity trumps hydrophobicity. J Mol Biol 370:459–470
Fox JL (2013) Antimicrobial peptides stage a comeback. Nat Biotechnol 31:379–382
Gaczynska M, Osmulski PA, Gao Y, Post MJ, Simons M (2003) Proline- and arginine-rich peptides constitute a novel class of allosteric inhibitors of proteasome activity. Biochemistry (Mosc) 42:8663–8670
Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, Bernfield M (1994) Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci USA 91:11035–11039
Gammon K (2014) Drug discovery: leaving no stone unturned. Nature 509:S10–S12
Gazit E, Boman A, Boman HG, Shai Y (1995) Interaction of the mammalian antibacterial peptide cecropin P1 with phospholipid vesicles. Biochemistry (Mosc) 34:11479–11488
Gennaro R, Skerlavaj B, Romeo D (1989) Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect Immun 57:3142–3146
Gennaro R, Zanetti M, Benincasa M, Podda E, Miani M (2002) Pro-rich antimicrobial peptides from animals: structure, biological functions and mechanism of action. Curr Pharm Des 8:763–778
Ghosh JK, Shaool D, Guillaud P, Cicéron L, Mazier D, Kustanovich I, Shai Y, Mor A (1997) Selective cytotoxicity of dermaseptin S3 toward intraerythrocytic Plasmodium falciparum and the underlying molecular basis. J Biol Chem 272:31609–31616
Giuliani A, Pirri G, Nicoletto S (2007) Antimicrobial peptides: an overview of a promising class of therapeutics. Cent Eur J Biol 2:1–33
Hallock KJ, Lee D-K, Ramamoorthy A (2003) MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys J 84:3052–3060
Hancock REW (1997) Peptide antibiotics. Lancet 349:418–422
Hancock REW, Nijnik A, Philpott DJ (2012) Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol 10:243–254
He K, Ludtke SJ, Worcester DL, Huang HW (1996) Neutron scattering in the plane of membranes: structure of alamethicin pores. Biophys J 70:2659–2666
Hede K (2014) Antibiotic resistance: an infectious arms race. Nature 509:S2–S3
Henzler Wildman KA, Lee D-K, Ramamoorthy A (2003) Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry (Mosc) 42:6545–6558
Hernandez-Gordillo V, Geisler I, Chmielewski J (2014) Dimeric unnatural polyproline-rich peptides with enhanced antibacterial activity. Bioorg Med Chem Lett 24:556–559
Hilchie AL, Wuerth K, Hancock REW (2013) Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 9:761–768
Hsueh P-R (2012) Study for monitoring antimicrobial resistance trends (SMART) in the Asia-Pacific region, 2002–2010. Int J Antimicrob Agents 40:S1–S3
Ilić N, Novković M, Guida F, Xhindoli D, Benincasa M, Tossi A, Juretić D (2013) Selective antimicrobial activity and mode of action of adepantins, glycine-rich peptide antibiotics based on anuran antimicrobial peptide sequences. Biochim Biophys Acta 1828:1004–1012
Jenssen H, Hamill P, Hancock REW (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19:491–511
Kanthor R (2014) Diagnostics: detection drives defence. Nature 509:S14–S15
Kavanagh K, Dowd S (2004) Histatins: antimicrobial peptides with therapeutic potential. J Pharm Pharmacol 56:285–289
Kragol G, Lovas S, Varadi G, Condie BA, Hoffmann R, Otvos L Jr (2001) The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry (Mosc) 40:3016–3026
Kraus D, Peschel A (2006) Molecular mechanisms of bacterial resistance to antimicrobial peptides. Curr Top Microbiol Immunol 306:231–250
Lalatsa A, Schatzlein AG, Uchegbu IF (2014) Strategies to deliver peptide drugs to the brain. Mol Pharm 11:1081–1093
Lehrer R, Barton A, Daher K, Harwig S, Ganz T, Selsted M (1989) Interaction of human defensins with Escherichia coli mechanism of bactericidal activity. J Clin Invest 84:553
Li CY, Song YL (2010) Proline-rich domain of penaeidin molecule exhibits autocrine feature by attracting penaeidin-positive granulocytes toward the wound-induced inflammatory site. Fish Shellfish Immunol 29:1044–1052
Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW (1996) Membrane pores induced by magainin. Biochemistry (Mosc) 35:13723–13728
Marr AK, Gooderham WJ, Hancock REW (2006) Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol 6:468–472
Matsuzaki K (1999) Why and how are peptide–lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta 1462:1–10
Matsuzaki K, Murase O, Fujii N, Miyajima K (1996) An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry (Mosc) 35:11361–11368
Matsuzaki K, Sugishita K, Ishibe N, Ueha M, Nakata S, Miyajima K, Epand RM (1998) Relationship of membrane curvature to the formation of pores by magainin 2. Biochemistry (Mosc) 37:11856–11863
Naito A, Nagao T, Norisada K, Mizuno T, Tuzi S, Saitô H (2000) Conformation and dynamics of melittin bound to magnetically oriented lipid bilayers by solid-state 31P and 13C NMR spectroscopy. Biophys J 78:2405–2417
Narayanan S, Modak JK, Ryan CS, Garcia-Bustos J, Davies JK, Roujeinikova A (2014) Mechanism of Escherichia coli resistance to pyrrhocoricin. Antimicrob Agents Chemother 58:2754–2762
Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y (1999) Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J 341:501–513
Ostorhazi E, Holub MC, Rozgonyi F, Harmos F, Cassone M, Wade JD, Otvos L Jr (2011) Broad-spectrum antimicrobial efficacy of peptide A3-APO in mouse models of multidrug-resistant wound and lung infections cannot be explained by in vitro activity against the pathogens involved. Int J Antimicrob Agents 37:480–484
Ostorhazi E, Voros E, Nemes-Nikodem E, Wade JD, Otvos L (2013) Rapid systemic and local treatments with the antibacterial peptide dimer A3-APO and its monomeric metabolite eliminate bacteria and reduce inflammation in intradermal lesions infected with Propionibacterium acnes and meticillin-resistant Staphyloccus aureus. Int J Antimicrob Agents 42:537–543
Otvos L (2000) Antibacterial peptides isolated from insects. J Pept Sci 6:497–511
Otvos L, Otvos I, Rogers ME, Consolvo PJ, Condie BA, Lovas S, Bulet P, Blaszczyk-Thurin M (2000) Interaction between heat shock proteins and antimicrobial peptides. Biochemistry (Mosc) 39:14150–14159
Otvos L (2002) The short proline-rich antibacterial peptide family. Cell Mol Life Sci 59:1138–1150
Otvos L (2005) Antibacterial peptides and proteins with multiple cellular targets. J Pept Sci 11:697–706
Otvos L, Cudic M, Chua BY, Deliyannis G, Jackson DC (2004) An insect antibacterial peptide-based drug delivery system. Mol Pharm 1:220–232
Otvos L, Flick-Smith H, Fox M, Ostorhazi E, Dawson RM, Wade JD (2014) The designer proline-rich antibacterial peptide A3-APO prevents Bacillus anthracis mortality by deactivating bacterial toxins. Protein Pept Lett 21:374–381
Patrzykat A, Friedrich CL, Zhang L, Mendoza V, Hancock REW (2002) Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob Agents Chemother 46:605–614
Paulsen VS, Blencke H-M, Benincasa M, Haug T, Eksteen JJ, Styrvold OB, Scocchi M, Stensvåg K (2013) Structure–activity relationships of the antimicrobial peptide arasin 1—and mode of action studies of the N-terminal, proline-rich region. PLoS ONE 8:e53326
Pelillo C, Benincasa M, Scocchi M, Gennaro R, Tossi A, Pacor S (2014) Cellular internalization and cytotoxicity of the antimicrobial proline-rich peptide Bac7(1–35) in monocytes/macrophages, and its activity against phagocytosed Salmonella typhimurium. Protein Pept Lett 21:382–390
Peters BM, Shirtliff ME, Jabra-Rizk MA (2010) Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog 6:e1001067
Podda E, Benincasa M, Pacor S, Micali F, Mattiuzzo M, Gennaro R, Scocchi M (2006) Dual mode of action of Bac7, a proline-rich antibacterial peptide. Biochim Biophys Acta 1760:1732–1740
Pouny Y, Rapaport D, Mor A, Nicolas P, Shai Y (1992) Interaction of antimicrobial dermaseptin and its fluorescently labeled analogs with phospholipid membranes. Biochemistry (Mosc) 31:12416–12423
Powers J-PS, Hancock REW (2003) The relationship between peptide structure and antibacterial activity. Peptides 24:1681–1691
Pushpanathan M, Gunasekaran P, Rajendhran J (2013) Antimicrobial peptides: versatile biological properties. Int J Pept 2013:15
Rappocciolo E (2004) Antimicrobial peptides as carriers of drugs. Drug Discov Today 9:470
Reddy KVR, Yedery RD, Aranha C (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24:536–547
Rolland JL, Abdelouahab M, Dupont J, Lefevre F, Bachere E, Romestand B (2010) Stylicins, a new family of antimicrobial peptides from the Pacific blue shrimp Litopenaeus stylirostris. Mol Immunol 47:1269–1277
Rozgonyi F, Szabo D, Kocsis B, Ostorhazi E, Abbadessa G, Cassone M, Wade JD, Otvos L (2009) The antibacterial effect of a proline-rich antibacterial peptide A3-APO. Curr Med Chem 16:3996–4002
Runti G, MdC Lopez Ruiz, Stoilova T, Hussain R, Jennions M, Choudhury HG, Benincasa M, Gennaro R, Beis K, Scocchi M (2013) Functional characterization of SbmA, a bacterial inner membrane transporter required for importing the antimicrobial peptide Bac7(1–35). J Bacteriol 195:5343–5351
Sadler K, Eom KD, Yang J-L, Dimitrova Y, Tam JP (2002) Translocating proline-rich peptides from the antimicrobial peptide bactenecin 7. Biochemistry (Mosc) 41:14150–14157
Salomón RA, Farías RN (1992) Microcin 25, a novel antimicrobial peptide produced by Escherichia coli. J Bacteriol 174:7428–7435
Scheit KH, Reddy ESP, Bhargava PM (1979) Seminalplasmin is a potent inhibitor of E. coli RNA polymerase in vitro. Nature 279:728–731
Scocchi M, Tossi A, Gennaro R (2011) Proline-rich antimicrobial peptides: converging to a non-lytic mechanism of action. Cell Mol Life Sci 68:2317–2330
Shai Y (1995) Molecular recognition between membrane-spanning polypeptides. Trends Biochem Sci 20:460–464
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462:55–70
Shamova O, Brogden KA, Zhao C, Nguyen T, Kokryakov VN, Lehrer RI (1999) Purification and properties of proline-rich antimicrobial peptides from sheep and goat leukocytes. Infect Immun 67:4106–4111
Shen X, Ye G, Cheng X, Yu C, Altosaar I, Hu C (2010) Characterization of an abaecin-like antimicrobial peptide identified from a Pteromalus puparum cDNA clone. J Invertebr Pathol 105:24–29
Shi J, Ross CR, Chengappa MM, Sylte MJ, McVey DS, Blecha F (1996) Antibacterial activity of a synthetic peptide (PR-26) derived from PR-39, a proline-arginine-rich neutrophil antimicrobial peptide. Antimicrob Agents Chemother 40:115–121
Smith R, Separovic F, Bennett FC, Cornell BA (1992) Melittin-induced changes in lipid multilayers. A solid-state NMR study. Biophys J 63:469–474
Splith K, Neundorf I (2011) Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur Biophys J 40:387–397
Stalmans S, Wynendaele E, Bracke N, Knappe D, Hoffmann R, Peremans K, Polis I, Burvenich C, De Spiegeleer B (2014) Blood–brain barrier transport of short proline-rich antimicrobial peptides. Protein Pept Lett 21:399–406
Stensvag K, Haug T, Sperstad SV, Rekdal O, Indrevoll B, Styrvold OB (2008) Arasin 1, a proline-arginine-rich antimicrobial peptide isolated from the spider crab, Hyas araneus. Dev Comp Immunol 32:275–285
Stewart KM, Horton KL, Kelley SO (2008) Cell-penetrating peptides as delivery vehicles for biology and medicine. Org Biomol Chem 6:2242–2255
Subbalakshmi C, Sitaram N (1998) Mechanism of antimicrobial action of indolicidin. FEMS Microbiol Lett 160:91–96
Szabo D, Ostorhazi E, Binas A, Rozgonyi F, Kocsis B, Cassone M, Wade JD, Nolte O, Otvos L Jr (2010) The designer proline-rich antibacterial peptide A3-APO is effective against systemic Escherichia coli infections in different mouse models. Int J Antimicrob Agents 35:357–361
Tian W, Li B, Zhang X, Dang W, Wang X, Tang H, Wang L, Cao H, Chen T (2012) Suppression of tumor invasion and migration in breast cancer cells following delivery of siRNA against Stat3 with the antimicrobial peptide PR39. Oncol Rep 28:1362–1368
Végh AG, Nagy K, Bálint Z, Kerényi Á, Rákhely G, Váró G, Szegletes Z (2011) Effect of antimicrobial peptide-amide: indolicidin on biological membranes. J Biomed Biotechnol 2011
Wilson SS, Wiens ME, Smith JG (2013) Antiviral mechanisms of human defensins. J Mol Biol 425:4965–4980
Wong H, Bowie JH, Carver JA (1997) The solution structure and activity of caerin 1.1, an antimicrobial peptide from the Australian green tree frog, Litoria splendida. Eur J Biochem 247:545–557
Wu M, Maier E, Benz R, Hancock REW (1999) Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry (Mosc) 38:7235–7242
Yamaguchi S, Huster D, Waring A, Lehrer RI, Kearney W, Tack BF, Hong M (2001) Orientation and dynamics of an antimicrobial peptide in the lipid bilayer by solid-state NMR spectroscopy. Biophys J 81:2203–2214
Yamaguchi S, Hong T, Waring A, Lehrer RI, Hong M (2002) Solid-state NMR investigations of peptide–lipid interaction and orientation of a β-sheet antimicrobial peptide, protegrin. Biochemistry (Mosc) 41:9852–9862
Yang L, Weiss TM, Lehrer RI, Huang HW (2000) Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys J 79:2002–2009
Yang L, Harroun TA, Weiss TM, Ding L, Huang HW (2001) Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J 81:1475–1485
Yu P-L, Cross ML, Haverkamp RG (2010) Antimicrobial and immunomodulatory activities of an ovine proline/arginine-rich cathelicidin. Int J Antimicrob Agents 35:288–291
Zahn M, Berthold N, Kieslich B, Knappe D, Hoffmann R, Sträter N (2013) Structural studies on the forward and reverse binding modes of peptides to the chaperone DnaK. J Mol Biol 425:2463–2479
Zahn M, Kieslich B, Berthold N, Knappe D, Hoffmann R, Strater N (2014) Structural identification of DnaK binding sites within bovine and sheep bactenecin Bac7. Protein Pept Lett 21:407–412
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zhang R, Eggleston K, Rotimi V, Zeckhauser RJ (2006) Antibiotic resistance as a global threat: evidence from China, Kuwait and the United States. Glob Health 2:6
Zhang L, Falla TJ (2006) Antimicrobial peptides: therapeutic potential. Expert Opin Pharmacother 7:653–663
Zhou Y, Chen WN (2011) iTRAQ-coupled 2-D LC–MS/MS analysis of membrane protein profile in Escherichia coli incubated with apidaecin IB. PLoS ONE 6:e20442
Zhu Y-G, Johnson TA, Su J-Q, Qiao M, Guo G-X, Stedtfeld RD, Hashsham SA, Tiedje JM (2013) Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci USA 110:3435–3440
Acknowledgments
We acknowledge partial support of the studies undertaken in the authors’ laboratory by the Australian Research Council (DP150103522) to MAH and JDW and the National Health and Medical Research Council (NHMRC) Grant APP1029878 to NMOBS. JDW is an NHMRC (Australia) Principal Research Fellow. Research at the FNI was supported by the Victorian Government’s Operational Infrastructure Support Program.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The manuscript does not contain clinical studies or patient data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Tailhades, J., O’Brien-Simpson, N.M. et al. Proline-rich antimicrobial peptides: potential therapeutics against antibiotic-resistant bacteria. Amino Acids 46, 2287–2294 (2014). https://doi.org/10.1007/s00726-014-1820-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1820-1