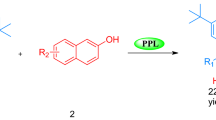
Abstract
A cascade reaction between aldehydes and indole catalyzed by lipase from porcine pancreas Type II (PPL) in solvent mixture at 50 °C was reported for the first time. Some control experiments had been designed to demonstrate that the PPL was responsible for the cascade reaction. After the optimization of the stepwise process, a series of bis(indolyl)alkanes were prepared in moderate to excellent yields under the catalysis of PPL.



Similar content being viewed by others
References
Aleu J, Bustillo AJ, Hernandez-Galan R, Collado IG (2006) Biocatalysis applied to the synthesis of agrochemicals. Curr Org Chem 10(16):2037–2054
Azizi N, Torkian L, Saidi M (2007) Highly efficient synthesis of bis(indolyl)methanes in water. J Mol Catal A: Chem 275(1–2):109–112
Babtie A, Tokuriki N, Hollfelder F (2010) What makes an enzyme promiscuous? Curr Opin Chem Biol 14(2):200–207
Bandgar BP, Shaikh KA (2003) Molecular iodine-catalyzed efficient and highly rapid synthesis of bis(indolyl)methanes under mild conditions. Tetrahedron Lett 44(9):1959–1961
Branneby C, Carlqvist P, Hult K, Brinck T, Berglund P (2004) Aldol additions with mutant lipase: analysis by experiments and theoretical calculations. J Mol Catal B Enzym 31(4–6):123–128
Cai JF, Guan Z, He YH (2011) The lipase-catalyzed asymmetric C–C Michael addition. J Mol Catal B Enzym 68(3–4):240–244
Carlqvist P, Svedendahl M, Branneby C, Hult K, Brinck T, Berglund P (2005) Exploring the active-site of a rationally redesigned lipase for catalysis of michael-type additions. ChemBioChem 6(2):331–336
Chakrabarty M, Ghosh N, Basak R, Harigaya Y (2002) Dry reaction of indoles with carbonyl compounds on montmorillonite K10 clay: a mild, expedient synthesis of diindolylalkanes and vibrindole A. Tetrahedron Lett 43(22):4075–4078
Chakrabarty M, Ghosh N, Basak R, Harigaya Y (2004) A facile and efficientsynthesis of 2,2-bis(3′/2′-indolyl)ethylamines and three bisindolic natural products. Synthetic Commun 34(3):421–434
Chen XA, Liu BK, Kang H, Lin XF (2011) A tandem aldol condensation/dehydration co-catalyzed by acylase and N-heterocyclic compounds in organic media. J Mol Catal B Enzym 68(1):71–76
Das PJ, Das J (2012) Synthesis of aryl/alkyl(2,2′-bis-3-methylindolyl)methanes and aryl(3,3′-bis indolyl)methanes promoted by secondary amine based ionic liquids and microwave irradiation. Tetrahedron Lett 53(35):4718–4720
Feng XW, Li C, Wang N, Li K, Zhang WWi, Wang Z et al (2009) Lipase-catalysed decarboxylative aldol reaction and decarboxylative Knoevenagel reaction. Green Chem 11(12):1933–1936
Firouzabadi H, Iranpoor Nr, Jafari AA (2006) Aluminumdodecatungstophosphate (AlPW12O40), a versatile and a highly water tolerant green lewis acid catalyzes efficient preparation of indole derivatives. J Mol Catal A: Chem 244(1–2):168–172
Fukuyama T, Chen XQ (1994) Stereocontrolled synthesis of (-)-Hapalindole G. J Am Chem Soc 116(7):3125–3126
Gatti-Lafranconi P, Hollfelder F (2013) Flexibility and reactivity in promiscuous enzymes. ChemBioChem 14(3):285–292
Gruber-Khadjawi M, Purkarthofer T, Skranc W, Griengl H (2007) Hydroxynitrile lyase-catalyzed enzymatic nitroaldol (henry) reaction. Adv Synth Catal 349(8–9):1445–1450
Guillermo PC, Jose-Guadalupe GE, Jose-Luis GR, Cecilio AT (2003) Infrared-assisted eco-friendly selective synthesis of diindolylmethanes. Green Chem 5(3):337–339
Guinchard X, Vallée Y, Denis J (2007) Total synthesis of marine sponge bis(indole) alkaloids of the topsentin class. J Org Chem 72(10):3972–3975
Hagiwara H, Sekifuji M, Hoshi T, Qiao K, Yokoyama C (2007) Synthesis of bis(indolyl)methanes catalyzed by acidic ionic liquid immobilized on silica (ILIS). Synlett 08:1320–1322
He YH, Hu W, Guan Z (2012) Enzyme-catalyzed direct three-component aza-diels-alder reaction using hen egg white lysozyme. J Org Chem 77(1):200–207
Hibino S, Choshi T (2001) Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat Prod Rep 18(1):66–87
Hu W, Guan Z, Deng X, He YH (2012) Enzyme catalytic promiscuity: the papain-catalyzed Knoevenagel reaction. Biochimie 94(3):656–661
Hult K, Berglund P (2007) Enzyme promiscuity: mechanism and applications. Trends Biotechnol 25(5):231–238
Humble MS, Berglund P (2011) Biocatalytic promiscuity. Eur J Org Chem 19:3391–3401
Ji SJ, Zhou MF, Gu DG, Wang SY, Loh TP (2003) Efficient synthesis of bis(indolyl)methanes catalyzed by lewis acids in ionic liquids. Synlett 13:2077–2079
Ji SJ, Zhou MF, Gu DG, Jiang ZQ, Loh TP (2004) Efficient Fe-III-catalyzed synthesis of bis(indolyl)methanes in ionic liquids. Eur J Org Chem 7:1584–1587
Jon-Paul-Selvam J, Srinivasulu M, Suryakiran N, Suresh V, Malla-Reddy S, Venkateswarlu Y (2008) Lanthanum(III) nitrate hexahydrate: a versatile reagent for the synthesis of bis(indolyl)methanes under solvent-Free conditions. Synthetic Commun 38(11):1760–1767
Kamble VT, Bandgar BP, Bavikar SN (2007a) Highly efficient synthesis of bis(indolyl)methanes catalyzed by sodium tetrafluoroborate. Chin J Chem 25(1):13–15
Kamble VT, Kadam KR, Joshi NS, Muley DB (2007b) HClO4–SiO2 as a novel and recyclable catalyst for the synthesis of bis-indolylmethanes and bis-indolylglycoconjugates. Catal Commun 8(3):498–502
Kantam ML, Aziz K, Likhar PR (2004) Bis(cyclopentadienyl)zirconium dichloride for alkylation of heteroaromatics and synthesis of bis(indolyl)methanes. Catalysis Lett 98(2–3):117–121
Kantam ML, Laha S, Yadav J, Sreedhar B (2006) Friedel–Crafts alkylation of indoles with epoxides catalyzed by nanocrystalline titanium(IV) oxide. Tetrahedron Lett 47(35):6213–6216
Karthik M, Tripathi AK, Gupta NM, Palanichamy M, Murugesan V (2004) Zeolite catalyzed electrophilic substitution reaction of indoles with aldehydes: synthesis of bis(indolyl)methanes. Catal Commun 5(7):371–375
Kazlauskas RJ (2005) Enhancing catalytic promiscuity for biocatalysis. Curr Opin Chem Biol 9(2):195–201
Khalafi-Nezhad A, Parhami A, Zare A, Zare ARM, Hasaninejad A, Panahi F (2008) Trityl chloride as a novel and efficient organic catalyst for room temperature preparation of bis(indolyl)methanes under solvent-free conditions in neutral media. Synthesis 4:617–621
Kidwai M, Bura N, Mishra NK (2011) Niobium(V) pentachloride-catalyzed efficient and highly rapid synthesis of bis(indolyl)methanes under mild conditions. Indian J Chem B 50(2):229–232
Kitazume T, Ikeya T, Murata K (1986) Synthesis of optically active trifluorinated compounds: asymmetric Michael addition with hydrolytic enzymes. J Chem Soc, Chem Commun(17):1331–1333
Klossowski S, Wiraszka B, Berlozecki S, Ostaszewski R (2013) Model studies on the first enzyme-catalyzed ugi reaction. Org Lett 15(3):566–569
Koshima H, Matsusaka W (2002) N-bromosuccinimide catalyzed condensations of indoles with carbonyl compounds under solvent-free conditions. J Heterocycl Chem 39(5):1089–1091
Krishnammagari SK, Chinnapareddy BR, Balam SK, Kambam S, Cirandur SR (2012) Micelle promoted synthesis of bis-(indolyl)methanes. Lett Org Chem 9(4):294–299
Lei P, Abdelrahim M, Cho SD, Liu SX, Chintharlapalli S, Safe S (2008) 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-jun N-terminal kinase. Carcinogenesis 29(6):1139–1147
Li C, Feng XW, Wang N, Zhou YY, Yu XQ (2008) Biocatalytic promiscuity: the first lipase-catalysed asymmetric aldol reaction. Green Chem 10(6):616–618
Liu ZQ, Liu BK, Wu Q, Lin XF (2011) Diastereoselective enzymatic synthesis of highly substituted 3,4-dihydropyridin-2-ones via domino Knoevenagel condensation-Michael addition-intramolecular cyclization. Tetrahedron 67(50):9736–9740
Lou FW, Xu JM, Liu BK, Wu Q, Pan Q, Lin XF (2007) Highly selective anti-Markovnikov addition of thiols to vinyl ethers under solvent- and catalyst-free conditions. Tetrahedron Lett 48(50):8815–8818
Lou FW, Liu BK, Wu Q, Lv DS, Lin XF (2008) Candida antarctica lipase B (CAL-B)-catalyzed carbon-sulfur bond addition and controllable selectivity in organic media. Adv Synth Catal 350(13):1959–1962
Lou FW, Liu BK, Wang JL, Pan Q, Lin XF (2009) Controllable enzymatic Markovnikov addition and acylation of thiols to vinyl esters. J Mol Catal B Enzym 60(1–2):64–68
Lounasmaa M, Tolvanen A (2000) Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat Prod Rep 17(2):175–191
Ma SM, Yu SC, Peng ZH (2005) Sc(OTf)3-catalyzed efficient synthesis of β,β-bis(indolyl) ketones by the double indolylation of acetic acid 2-methylene-3-oxobutyl ester. Org Biomol Chem 3(10):1933–1936
Martínez R, Espinosa A, Tárraga A, Molina P (2008) Bis(indolyl)methane derivatives as highly selective colourimetric and ratiometric fluorescent molecular chemosensors for Cu2+ cations. Tetrahedron 64(9):2184–2191
Mehrazma S, Azizi N, Saidi MR (2006) Clean and facile condensations reaction of indoles and carbonyl compounds under solvent-free conditions. Lett Org Chem 3(2):161–164
Mohit LD, Pulak JB (2006) An efficient and clean synthesis of bis(indolyl)methanes in a protic solvent at room temperature. Tetrahedron Lett 47(9):1441–1443
Mona HS (2007) Titania (TiO2)-catalyzed expedient, solventless and mild synthesis of bis(indolyl)methanes. Acta Chim Slov 54(2):354–359
Nadkarni SV, Gawande MB, Jayaram RV, Nagarkar JM (2008) Synthesis of bis(indolyl)methanes catalyzed by surface modified zirconia. Catal Commun 9(8):1728–1733
Niknam K, Zolfigol MA, Sadabadi T, Nejati A (2006) Preparation of indolylmethanes catalyzed by metal hydrogen sulfates. J Iran Chem Soc 3(4):318–322
Pollard DJ, Woodley JM (2007) Biocatalysis for pharmaceutical intermediates: the future is now. Trends Biotechnol 25(2):66–73
Pradhan PK, Dey S, Giri VS, Jaisankar P (2005) InCl3-HMTA as a methylene donor: one-pot synthesis of diindolylmethane (DIM) and its derivatives. Synthesis(11):1779–1782
Purkarthofer T, Gruber K, Gruber-Khadjawi M, Waich K, Skranc W, Mink D et al (2006) A Biocatalytic Henry Reaction—The Hydroxynitrile Lyase from Hevea brasiliensis Also Catalyzes Nitroaldol Reactions. Angew Chem Int Ed 45(21):3454–3456
Safe S, Papineni S, Chintharlapalli S (2008) Cancer chemotherapy with indole-3-carbinol, bis(3′-indolyl)methane and synthetic analogs. Cancer Lett 269(2):326–338
Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts M, Witholt B (2001) Industrial biocatalysis today and tomorrow. Nature 409(6817):258–268
Serafimov M Jr, Gillingham D, Kuster S, Hilvert D (2008) The Putative Diels–Alderase Macrophomate Synthase is an Efficient Aldolase. J Am Chem Soc 130(25):7798–7799
Sharma GVM, Janardhan-Reddy J, Sree-Lakshmi P, Radha-Krishna P (2004) A versatile and practical synthesis of bis(indolyl)methanes/bis(indolyl)glycoconjugates catalyzed by trichloro-1,3,5-triazine. Tetrahedron Lett 45(41):7729–7732
Shiri M, Zolfigol MA, Kruger HG, Tanbakouchian Z (2009) Bis- and trisindolylmethanes (Bims and Tims). Chem Rev 110(4):2250–2293
Silveira CC, Mendes SR, Líbero FM, ErJ Lenardão, Perin G (2009) Glycerin and CeCl3·7H2O: a new and efficient recyclable medium for the synthesis of bis(indolyl)methanes. Tetrahedron Lett 50(44):6060–6063
Svedendahl M, Hult K, Berglund P (2005) Fast carbon–carbon bond formation by a promiscuous lipase. J Am Chem Soc 127(51):17988–17989
Taglieber A, Hoebenreich H, Carballeira JD, Mondiere R, Reetz MT (2007) Alternate-site enzyme promiscuity. Angew Chem Int Ed 46(45):8597–8600
Tayebee R, Amini MM, Nehzat F, Sadeghi O, Armaghan M (2013) H5PW10V2O40/pyridino-SBA-15 as a highly recyclable, robust and efficient inorganic-organic hybrid material for the catalytic preparation of bis(indolyl)methanes. J Mol Catal A: Chem 366:140–148
Torre O, Alfonso I, Gotor V (2004) Lipase catalysed Michael addition of secondary amines to acrylonitrile. Chem Commun 7(15):1724–1725
Wang SY, Ji SJ (2008) Facile synthesis of bis(indolyl)methanes catalyzed by ferric dodecyl sulfonate [Fe(DS)3] in water at room temperature. Synthetic Commun 38(8):1291–1298
Wang JL, Li X, Xie HY, Liu BK, Lin XF (2010) Hydrolase-catalyzed fast Henry reaction of nitroalkanes and aldehydes in organic media. J Biotechnol 145(3):240–243
Wang JL, Liu BK, Yin C, Wu Q, Lin XF (2011) Candida antarctica lipase B-catalyzed the unprecedented three-component Hantzsch-type reaction of aldehyde with acetamide and 1,3-dicarbonyl compounds in non-aqueous solvent. Tetrahedron 67(14):2689–2692
Wu WB, Wang N, Xu JM, Wu Q, Lin XF (2005) Penicillin G acylase catalyzed Markovnikov addition of allopurinol to vinyl ester. Chem Commun 18:2348–2350
Wu WB, Xu JM, Wu Q, Lv DS, Lin XF (2006) Promiscuous acylases-catalyzed markovnikov addition of N-heterocycles to vinyl esters in organic media. Adv Synth Catal 348(4–5):487–492
Wu Q, Liu BK, Lin XF (2010) Enzymatic promiscuity for organic synthesis and cascade process. Curr Org Chem 14(17):1966–1988
Xia M, Wang SH, Yuan WB (2004) Lewis acid catalyzed electrophilic substitution of indole with aldehydes and Schiff’s bases under microwave solvent-free irradiation. Synthetic Commun 34(17):3175–3182
Xl Mi, Luo SZ, He JQ, Cheng JP (2004) Dy(OTf)3 in ionic liquid: an efficient catalytic system for reactions of indole with aldehydes/ketones or imines. Tetrahedron Lett 45(23):4567–4570
Xu JM, Zhang F, Liu BK, Wu Q, Lin XF (2007) Promiscuous zinc-dependent acylase-mediated carbon–carbon bond formation in organic media. Chem Commun 20:2078–2080
Xu HF, Zi Y, Xu XP, Wang SY, Ji SJ (2013) TFA-catalyzed C–N bond activation of enamides with indoles: efficient synthesis of 3,3-bisindolylpropanoates and other bisindolylalkanes. Tetrahedron 69(5):1600–1605
Xue Y, Li LP, He YH, Guan Z (2012) Protease-catalysed direct asymmetric Mannich reaction in organic solvent. Sci Rep 2:761
Yadav JS, Reddy BVS, Murthy C, Kumar GM, Madan C (2001) Lithium perchlorate catalyzed reactions of indoles: an expeditious synthesis of bis(indolyl)methanes. Synthesis 5:783–787
Yang YL, Xie ZF, Wang JD (2011) CrCl3·6H2O/hydrogenated bis-schiff base as a new efficient catalyst system for synthesis of bis(indoly) methane. Chin J Chem 29(10):2091–2096
Zhang ZH, Yin L, Wang YM (2005) An efficient and practical process for the synthesis of bis(indolyl)methanes catalyzed by zirconium tetrachloride. Synthesis 12:1949–1954
Zolfigol M, Salehi P, Shiri M, Sayadi A, Abdoli A, Keypour H et al (2008) A simple and efficient route for the synthesis of di and tri(bis(indolyl) methanes) as new triarylmethanes. Mol Divers 12(3–4):203–207
Acknowledgments
The authors gratefully acknowledge generous financial support from National Natural Science Foundation of China (No. 21072172, 21272208) and the Zhejiang Provincial Natural Science Foundation (Project No. 2010-Z4090225).
Conflict of interest
We declare that we have no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xiang, Z., Liu, Z., Chen, X. et al. Biocatalysts for cascade reaction: porcine pancreas lipase (PPL)-catalyzed synthesis of bis(indolyl)alkanes. Amino Acids 45, 937–945 (2013). https://doi.org/10.1007/s00726-013-1547-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1547-4