Abstract
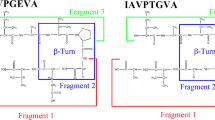
This study presents a design of a highly potent and competitive inhibitory peptide for 3-hydroxy-3-methylglutaryl CoA reductase (HMGR). HMGR is the major regulatory enzyme of cholesterol biosynthesis and the target enzyme of many investigations aimed at lowering the rate of cholesterol biosynthesis. In previous studies, the two hypocholesterolemic peptides (LPYP and IAVPGEVA) were isolated and identified from soy protein. Based on these peptide sequences, a number of peptides were designed previously by using the correlation between the conformational flexibility and bioactivity. The design method that was applied in previous studies was slightly modified for the purpose of the current research and 12 new peptides were designed and synthesized. Among all peptides, SFGYVAE showed the highest ability to inhibit HMGR. A kinetic analysis revealed that this peptide is a competitive inhibitor of HMG-CoA with an equilibrium constant of inhibitor binding (K i) of 12 ± 0.4 nM. This is an overall 14,500-fold increase in inhibitory activity compared to the first isolated LPYP peptide from soybeans. Conformational data support a conformation of the designed peptides close to the bioactive conformation of the previously synthesized active peptides.







Similar content being viewed by others
References
Becker OM (1997) Geometric versus topological clustering: an insight into conformational mapping. Proteins 27:213–226
Beker OM, Levy Y, Ravitz O (2000) Flexibility, conformation space, and bioactivity. J Phys Chem 104:2123–2135
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 72:248–254
Burkert U, Allinger NL (1982) Molecular mechanics. ACS Monograph N. 177. ACS, Washington
Deedwania P, Singh V, Davidson MH (2009) Low high-density lipoprotein cholesterol and increased cardiovascular disease risk: an analysis of statin clinical trials. Am J Cardiol 104:3E–9E
Dewar MJS, Zoebish EG, Healy EF, Stewart JJP (1985) Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. J Am Chem Soc 107:3902–3909
Endo A (1992) The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res 33:1569–1582
Fields CG, Lloyd DH, Macdonald RL, Otteson KM, Noble RL (1991) HBTU activation for automated Fmoc solid-phase peptide synthesis. Pept Res 4:95–101
Fisher R (1935) The design of experiments. Oxford University Press, Oxford
Gunasekaran K, Gomathi L, Ramakrishman C, Chandrasekhar J, Balaram P (1998) Conformational interconversions in peptide β-turns: analysis of turns in proteins and computational estimates of barriers. J Mol Biol 284:1505–1516
Istvan E (2003) Statin inhibition of HMG-CoA reductase: a 3-dimensional view. Atheroscler Suppl 4:3–8
Johnson WC Jr (1990) Protein secondary structure and circular dichroism: a practical guide. Protein Struct Funct Genet 7:205–214
Kalazsi A, Farkas O (2003) Lead conformer prediction based on a library of flexible molecules. J Mol Struct (Theochem) 666–667:645–649
Karle IL, Karle J (1963) An application of a new phase determination procedure to the structure of cyclo(hexaglycyl) hemihydrate. Acta Crystallogr 16:969–975
Kollman PA (1996) Advances and continuing challenges in achieving realistic and predictive simulations of the properties of organic and biological molecules. Acc Chem Res 29:461–469
Krittanai C, Johnson WC Jr (1997) Correcting the circular dichroism spectra of peptides for contributions of absorbing side chains. Anal Biochem 253:57–64
Kwon DY, Oh SW, Lee JS, Yang HJ, Lee SH, Lee JH, Lee YB, Sohn HS (2002) Amino acid substitution of hypocholesterolemic peptides originated from glycinin hydrolyzate. Food Sci Biotechnol 11:55–61
Pak VV, Koo M, Kasimova TD, Kwon DY (2004) Conformation analysis of Ile-Ala-Val-Pro peptide and its derivatives by circular dichroism. Chem Nat Compd 40:398–404
Pak VV, Koo M, Lee N, Lee JS, Kasimova TD, Kwon DY (2005a) Isolation and identification of hypocholesterolemic peptide from 11S globulin of soy protein. Chem Nat Compd 41:710–714
Pak VV, Koo M, Lee N, Lee JS, Kasimova TD, Kwon DY (2005b) Structure-activity relationship of Ile-Ala-Val-Pro peptide and its derivatives by using semi-empirical AM1 method. Chem Nat Compd 41:454–460
Pak VV, Kim SH, Koo M, Lee N, Shakhidoyatov KM, Kwon DY (2006) Peptide design of a competitive inhibitor for HMG-CoA reductase based on statin structure. Biopolymers (Pept Sci) 84:586–594
Pak VV, Koo M, Yun LM, Kwon DY (2007) Recognized sequence and conformation in design of linear peptides as a competitive inhibitor for HMG-CoA reductase. J Mol Recognit 20:197–203
Pak VV, Koo M, Kim MJ, Yang HJ, Yun L, Kwon DY (2008) Modeling an active conformation for linear peptides and design of a competitive inhibitor for HMG-CoA reductase. J Mol Recognit 21:224–232
Payne JW, Marshall NJ, Grail BM, Gupta S (2002) Conformer profiles and biological activities of peptides. Curr Org Chem 6:1221–1246
Perkin Elmer (1995) Introduction to cleavage techniques. Perkin Elmer Cooperation, Foster City
Rader DJ (2003) Regulation of reverse cholesterol transport and clinical implications. Am J Cardiol 92:42–49
Segel IH (1976) Biochemical calculations. Wiley, New York
Voet D, Voet JG (1990) Biochemistry. Wiley, New York, pp 330–340
Acknowledgments
This work was supported by Korea Food Research Institute, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pak, V.V., Koo, M., Kwon, D.Y. et al. Design of a highly potent inhibitory peptide acting as a competitive inhibitor of HMG-CoA reductase. Amino Acids 43, 2015–2025 (2012). https://doi.org/10.1007/s00726-012-1276-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1276-0