Abstract
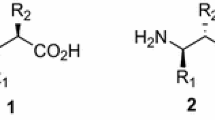
Constrained enantiopure bicyclic β-amino acids derived from the asymmetric Diels–Alder reaction of the (R)-benzyl-4-(3-acryloyloxy-4,4-dimethyl-2-oxopyrrolidin-1-yl)-benzoate and the 1-(benzyloxycarbonylamino)cyclohexadiene provide original templates for the construction of new rigid enantiopure 1,3-amino alcohols.






Similar content being viewed by others
References
Ager DJ, Prakash I, Schaad DR (1996) 1, 2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem Rev 96:835–876
Akkari R, Calmès M, Escale F, Iapichella J, Rolland M, Martinez J (2004) (R)- or (S)-4-(3-hydroxy-4, 4-dimethyl-2-oxopyrrolidin-1-yl) benzoic acid as a new chiral auxiliary for solid phase asymmetric Diels–Alder reactions. Tetrahedron Asymmetr 15:2515–2525
Balazs AS, Szakonyi Z, Fülöp F (2007) Synthesis of alicyclic N-substituted 1, 3-aminoalcohols via 1, 3-oxazines. J Heterocyclic Chem 44:403–406
Barluenga J, Viado AL, Aguilar E, Fustero S, Olano B (1993) 1, 3-Amino alcohols from 4-amino-1-azadienes. Diastereo- and enantioselective approach to the four diastereoisomers of the N-terminal amino acid component of nikkomycins B and BX. J Org Chem 58:5972–5975
Bartoli G, Cimarelli C, Marcantoni E, Palmieri G, Petrini M (1994) Chemo- and diastereoselective reduction of beta-enamino esters: a convenient synthesis of both cis- and trans-γ-amino alcohols and β-amino esters. J Org Chem 59:5328–5335
Binder CM, Bautista A, Zaidlewicz M, Krzemiński MP, Oliver A, Singaram B (2009) Stereoselectivity in the dialkylzinc reaction using (−)-β-pinene derived amino alcohol chiral auxiliaries. J Org Chem 74:2337–2343
Blaser HU (1992) The chiral pool as a source of enantioselective catalysts and auxiliaries. Chem Rev 92:935–952
Calmès M, Didierjean C, Martinez J, Songis O (2005) (R)-(4-(Benzyloxycarbonylphenyl)-3-hydroxy-4, 4-dimethyl-2-pyrrolidinone) acrylate derivative as a chiral dienophile for the synthesis of enantiopure 2-aminocyclohexane carboxylic acids. Tetrahedron Asymmetr 16:2173–2178
Corey EJ, Helal CJ (1998) Reduction of carbonyl compounds with chiral oxazaborolidine catalysts: A new paradigm for enantioselective catalysis and a powerful new synthetic sethod. Angew Chem Int Ed Engl 37:1986–2012
Corey EJ, Bakshi RK, Shibata S (1987) Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications. J Am Chem Soc 109:5551–5553
Delair P, Einhorn C, Einhorn J, Luche JL (1994) Synthesis of beta-amino alcohols derived from l-valine. J Org Chem 59:4680–4682
Deloux L, Screbnik M (1993) Asymmetric boron-catalyzed reactions. Chem Rev 93:763–784
Didier E, Loubinoux B, Ramos Tombo GM, Rihs G (1991) Chemo-enzymatic synthesis of 1, 2- and 1, 3-aminoalcohols and their use in the enantioselective reduction of acetophenone and anti-acetophenone oxime methyl ether with borane. Tetrahedron 47:4941–4958
Fülöp F (2001) The chemistry of 2-aminocycloalkanecarboxylic acids. Chem Rev 101:2181–2204
Fülöp F, Szakonyi Z, Bernath G, Sohar P (1997) Saturated heterocycles: synthesis of 2, 4-dioxo and 4-oxo-2-thioxo derivatives of octahydrocyclopenta[d]pyrimidines. J Heterocyclic Chem 34:1211–1214
Garcia Martinez A, Teso Vilar E, Garcıa Fraile A, de la Moya Cerero S, Martinez Ruiz P, Chicharro Villas P (2002) Bridgehead-norbornane-derived β-amino alcohol catalysts: structural factors influencing the chirality transfer. Tetrahedron Asymmetr 13:1–4
Gyonfalvi S, Szakonyi Z, Fülöp F (2003) Synthesis and transformation of novel cyclic β-amino acid derivatives from (+)-3-carene. Tetrahedron Asymmetr 14:3965–3972
Hobuss D, Baro A, Laschat S, Frey W (2008) Catalytic enantioselective borane reduction of arylketones with pinene-derived amino alcohols. Tetrahedron 64:1635–1640
Khalil EM, Subasinghe NL, Johnson RL (1996) An efficient and high yield method for the N-tert-butyloxycarbonyl protection of sterically hindered amino acids. Tetrahedron Lett 37:3441–3444
Kiss L, Fülöp F (2009) Selective syntheses of functionalized cyclic β-amino acids via transformation of the ring C-C double bonds. Synlett 9:1302–1314
Kiss L, Forró E, Fülöp F (2009) Synthesis of carbocyclic β-amino acids. In: Hughes AB (ed) Amino acids, peptides and proteins in organic chemistry: origins and synthesis of amino acids, vol 1. Wiley-VCH, Weinheim, pp 367–409
Kitamura M, Suga S, Kawai K, Noyori R (1986) Catalytic asymmetric induction. Highly enantioselective addition of dialkylzincs to aldehydes. J Am Chem Soc 108:6071–6072
Kivelä H, Klika KD, Szabo A, Stajer G, Pihlaja K (2003) Structures of saturated 5H-pyrrolo[1,2-a][3,1]benzoxazin-1(2H)-ones prepared from 4-oxopentanoic acid and cyclic amino alcohols. Eur J Org Chem 1879–1886
Kivelä H, Zalan Z, Tahtinen P, Sillanpää R, Fülöp F, Pihlaja K (2005) Synthesis and conformational analysis of saturated 3, 1, 2-benzoxazaphosphine 2-oxides. Eur J Org Chem 9:1189–1200
Kochi T, Tang TP, Ellman JA (2003) Development and application of a new general method for the asymmetric synthesis of syn- and anti-1, 3-amino alcohols. J Am Chem Soc 125:11276–11282
Kossenjans M, Martens J (1998) Synthesis of C 2-symmetrical bis-β-amino alcohols from (R)-cysteine and their application in enantioselective catalysis. Tetrahedron Asymmetr 9:1409–1417
Kossenjans M, Martens J (1999) Highly stereoselective synthesis of 1, 3-aminoalcohols via Mannich reactions. Tetrahedron Asymmetr 10:3409–3416
Krzemiński MP, Wojtczak A (2005) Chiral terpene auxiliaries Part 1: Highly enantioselective reduction of ketones with borane catalyzed by an oxazaborolidine derived from (-)-β-pinene. Tetrahedron Lett 46:8299–8302
Krzemiński MP, Zaidlewicz M (2003) Asymmetric reduction of ketoxime derivatives and N-alkylketimines with borane-oxazaborolidine adducts. Tetrahedron Asymmetr 14:1463–1466
Laczkowski K, Kmieciak A, Kozakiewicz A (2009) Stereoselective synthesis of new monoterpene β-amino alcohols. Tetrahedron Asymmetr 20:1487–1492
Lait SM, Rankic DA, Keay BA (2007) 1, 3-Aminoalcohols and their derivatives in asymmetric organic synthesis. Chem Rev 107:767–796
Li X, Yeung CH, Chan ASC, Yang TK (1999) New 1, 3-amino alcohols derived from ketopinic acid and their application in catalytic enantioselective reduction of prochiral ketones. Tetrahedron Asymmetr 10:759–763
Masui M, Shiori T (1998) Stereoselective synthesis of 1, 2-amino alcohols by asymmetric borane reduction of α-oxoketoxime ethers. Tetrahedron Lett 39:5195–5198
McGeary RP (1998) Facile and chemoselective reduction of carboxylic acids to alcohols using BOP reagent and sodium borohydride. Tetrahedron Lett 39:3319–3322
Murai T, Sano H, Kawai H, Aso H, Shibahara F (2005) N-Thioacyl-1, 3-amino alcohols: synthesis via ring-opening of oxiranes with thioamide dianions and applications as key intermediates leading to stereochemically defined 5, 6-dihydro-4H–1, 3-oxazines and 1, 3-amino alcohols. J Org Chem 70:8148–8153
Oliveira L, Costa V (2004) An efficient synthesis of enantiopure (+)- and (−)-syn-1, 3-amino alcohols with the norbornane framework and their application in the asymmetric addition of ZnEt2 to benzaldehyde. Tetrahedron Asymmetr 15:2583–2590
Park K-H, Kurth MJ (2002) Cyclic amino acid derivatives. Tetrahedron 58:8629–8659
Raghavan S, Rajender A, Joseph SC, Rasheed MA, Kumar KR (2004) Regio- and stereoselective synthesis of 1, 3-aminoalcohol derivatives from allylamine derivatives via internal sulfinyl group participation. Tetrahedron Asymmetr 15:365–379
Reetz MT, Drewes MW, Schmitz A (1987) Stereoselective synthesis of β-amino alcohols from optically active α-amino acids. Angew Chem Int Ed Engl 26:1141–1143
Scarpi D, Occhiato EG, Guarna A (2009) Asymmetric addition of diethylzinc to aldehyde catalyzed by a camphor-derived β-amino alcohol. Tetrahedron Asymmetr 20:340–350
Songis O, Géant PY, Sautrey G, Martinez J, Calmès M (2008) Asymmetric Diels-Alder reaction of nonracemic acrylate bound to Rink resin with some aminodienes: a comparison of these reactions to their solution state analogues. Eur J Org Chem 308–318
Songis O, Didierjean C, Martinez J, Calmès M (2007) Asymmetric Diels-Alder cycloaddition of 1-aminocyclohexadiene to chiral acrylate: Synthesis of enantiopure bridgehead aminobicyclo[2.2.2]octane-2-carboxylic acid derivatives. Eur J Org Chem 3166–3172
Songis O, Didierjean C, Martinez J, Calmès M (2008b) The iodolactone approach to enantiopure oxiranes of constrained chiral cyclic β-amino acids. Tetrahedron Asymmetr 19:2135–2139
Szakonyi Z, Balazs A, Martinek TA, Fülöp F (2006) Enantioselective addition of ZnEt2 to aldehyde catalyzed by γ-amino alcohols derived from (+)- and (−)-α-pinène. Tetrahedron Asymmetr 17:199–204
Wu ZL, Wu HL, Wu PY, Uang BJ (2009) N-Substituent effects on the diethylzinc addition to benzaldehyde catalyzed by bicyclic 1, 4-aminoalcohols. Tetrahedron Asymmetr 20:1556–1560
Acknowledgments
The authors gratefully acknowledge the CNRS and the MESR for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
André, C., Calmès, M., Escale, F. et al. New 1,3-amino alcohols derived from enantiopure bridgehead β-aminobicyclo[2.2.2]oct-5-ene-2-carboxylic acids. Amino Acids 43, 415–421 (2012). https://doi.org/10.1007/s00726-011-1097-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1097-6