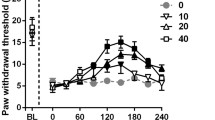
Abstract
Hypotaurine is an intermediate in taurine biosynthesis from cysteine in astrocytes. Although hypotaurine functions as an antioxidant and organic osmolyte, its physiological role in the central nervous system remains unclear. This study used behavioral assessments to determine whether hypotaurine influenced nociceptive transmission in acute, inflammatory, and neuropathic pain. The tail flick, paw pressure, and formalin tests were performed in male Sprague–Dawley rats to examine the effects of the intrathecal administration of hypotaurine (100, 200, 400, 600 μg) on thermal, mechanical, and chemical nociception. Chronic constriction injury (CCI) to the sciatic nerve was induced in the rats, and the electronic von Frey test and plantar test were performed to assess the effects on neuropathic pain. To determine which neurotransmitter pathway(s) was involved in the action of hypotaurine, in this study, we examined how the antagonists of spinal pain processing receptors altered the effect of 600 μg hypotaurine. To explore whether hypotaurine affected motor performance, the Rotarod test was conducted. Hypotaurine had antinociceptive effects on thermal, mechanical, and chemical nociception in the spinal cord. In CCI rats, hypotaurine alleviated mechanical allodynia and thermal hyperalgesia. These effects were reversed completely by pretreatment with an intrathecal injection of strychnine, a glycine receptor antagonist. Conversely, hypotaurine did not affect motor performance. This study demonstrated that intrathecal hypotaurine suppressed acute, inflammatory, and neuropathic pain. Hypotaurine may regulate nociceptive transmission physiologically by activating glycinergic neurons in the spinal cord, and it is a promising candidate for treating various pain states.






Similar content being viewed by others
References
Albrecht J, Schousboe A (2005) Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res 30:1615–1621 (Review)
Aruoma OI, Halliwell B, Hoey BM, Butler J (1998) The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem J 256:251–255
Banerjee R, Vitvitsky V, Garg SK (2008) The undertow of sulfur metabolism on glutamatergic neurotransmission. Trends Biochem Sci 33:413–419 (Review)
Belfer I, Davidson E, Ratner A, Beery E, Shir Y, Seltzer Z (1998) Dietary supplementation with the inhibitory amino acid taurine suppresses autotomy in HA rats. Neuroreport 9:3103–3107
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Betley JN, Wright CV, Kawaguchi Y, Erdélyi F, Szabó G, Jessell TM, Kaltschmidt JA (2009) Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 139:161–174
Bousquet M, Gibrat C, Ouellet M, Rouillard C, Calon F, Cicchetti F (2010) Cystamine metabolism and brain transport properties: clinical implications for neurodegenerative diseases. J Neurochem 114:1651–1658
Brand A, Leibfritz D, Hamprecht B, Dringen R (1998) Metabolism of cysteine in astroglial cells: synthesis of hypotaurine and taurine. J Neurochem 71:827–832
Coderre TJ, Fundytus ME, McKenna JE, Dalal S, Melzack R (1993) The formalin test: a validation of the weighted-scores method of behavioural pain rating. Pain 54:43–50
Enna SJ, McCarson KE (2006) The role of GABA in the mediation and perception of pain. Adv Pharmacol 54:1–27 (Review)
Gupta RC (2006) Taurine analogues and taurine transport: therapeutic advantages. Adv Exp Med Biol 583:449–467 (Review)
Haranishi Y, Hara K, Terada T, Nakamura S, Sata T (2010) The antinociceptive effect of intrathecal administration of glycine transporter-2 inhibitor ALX1393 in a rat acute pain model. Anesth Analg 110:615–621
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Huang W, Simpson RK (2000) Long-term intrathecal administration of glycine prevents mechanical hyperalgesia in a rat model of neuropathic pain. Neurol Res 22:160–164
Legendre P (2001) The glycinergic inhibitory synapse. Cell Mol Life Sci 58:760–793 (Review)
Lynch JW (2004) Molecular structure and function of the glycine receptor chloride channel. Physiol Rev 84:1051–1095 (Review)
Mehta TR, Dawson R Jr (2001) Taurine is a weak scavenger of peroxynitrite and does not attenuate sodium nitroprusside toxicity to cells in culture. Amino Acids 20:419–433
Okamoto K, Sakai Y (1981) Inhibitory actions of taurocyamine, hypotaurine, homotaurine, taurine and GABA on spike discharges of Purkinje cells, and localization of sensitive sites, in guinea pig cerebellar slices. Brain Res 206:371–386
Pellicer F, López-Avila A, Coffeen U, Manuel Ortega-Legaspi J, Angel RD (2007) Taurine in the anterior cingulate cortex diminishes neuropathic nociception: a possible interaction with the glycine(A) receptor. Eur J Pain 11:444–451
Randall LO, Selitto J (1957) A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn 4:409–419
Serrano MI, Serrano JS, Guerrero MR, Fernández A (1994) Role of GABAA and GABAB receptors and peripheral cholinergic mechanisms in the antinociceptive action of taurine. Gen Pharmacol 25:1123–1129
Silva MA, Cunha GM, Viana GS, Rao VS (1993) Taurine modulates chemical nociception in mice. Braz J Med Biol Res 26:1319–1324
Smullin DH, Schamber CD, Skilling SR, Larson AA (1990) A possible role for taurine in analgesia. Prog Clin Biol Res 351:129–132
Terada T, Hara K, Haranishi Y, Sata T (2011) Antinociceptive effect of intrathecal administration of taurine in rat models of neuropathic pain. Can J Anesth 58:630–637
Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992) The formalin test: an evaluation of the method. Pain 51:5–17
Vitvitsky V, Garg SK, Banerjee R (2011) Taurine biosynthesis by neurons and astrocytes. J Biol Chem 286:32002–32010
Yaksh TL, Rudy TA (1976) Chronic catheterization of the spinal subarachnoid space. Physiol Behav 17:1031–1036
Zeilhofer HU (2005) The glycinergic control of spinal pain processing. Cell Mol Life Sci 62:2027–2035 (Review)
Zeilhofer HU, Zeilhofer UB (2008) Spinal dis-inhibition in inflammatory pain. Neurosci Lett 437:170–174 (Review)
Zwingmann C, Leibfritz D (2005) Ammonia toxicity under hyponatremic conditions in astrocytes: de novo synthesis of amino acids for the osmoregulatory response. Neurochem Int 47:39–50
Acknowledgments
This study was supported in part by Grants-in-Aid for Research from the Ministry of Education, Science and Culture of Japan, (No. 19791095, 23592316 to K.H.; No. 23590732 to Y.H.; No. 21791481 to M.N.).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hara, K., Nakamura, M., Haranishi, Y. et al. Antinociceptive effect of intrathecal administration of hypotaurine in rat models of inflammatory and neuropathic pain. Amino Acids 43, 397–404 (2012). https://doi.org/10.1007/s00726-011-1094-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1094-9