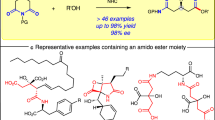
Abstract
(S)- and (R)-BIMBOL were efficient PT catalysts of asymmetric Michael addition of prochiral Ni–PBP–Gly (1) to acrylic esters and malonic esters to Ni–PBP–Δ-Ala (2) correspondingly. The salient feature of the catalysis is opposite configurations of Glu prepared via the two paths with BIMBOL of the same configuration and a perspective novel catalytic procedure for the synthesis of Gla derivatives.








Similar content being viewed by others
Abbreviations
- PBP:
-
N-(2-benzoylphenyl)pyridine-2-carboxamide
- Ni–PBP–Gly (1):
-
Ni(II) complex of a Schiff base of glycine with PBP
- Ni–PBP–Δ-Ala (2):
-
Ni(II) complex of Schiff base of dehydroalanine with PBP
- MOM:
-
Methoxymethylene group
- DCE:
-
Dichloroethane
- DCM:
-
Dichloromethane
- HMDSLi:
-
Lithium hexamethyldisilaside
- Gly:
-
Glycine
- Glu:
-
Glutamic acid
- Gla:
-
γ-Carboxyglutamic acid
- PTC:
-
Phase transfer catalysis
- TADDOL:
-
(2,2′-Dimethyl-1,3-dioxolan-4,5-diyl)bis(diphenylmethanol)
- NOBIN:
-
2′-Amino-[1,1′-binaphthalen]-2-ol
- iso-NOBIN:
-
8′-Amino-[1,1′-binaphthalen]-2-ol
- BINOL:
-
[1,1′-Binaphthalene]-2,2’-diol
- BIMBOL:
-
3,3′-Bis(hydroxydiphenylmethyl)-[1,1′-binaphthalene]-2,2′-diol
- ee:
-
Enantiomeric excess
- dr:
-
Diastereomers ratio
References
Belokon YN, Bespalova NB, Churkina TD, Císařová I, Ezernitskaya MG, Harutyunyan SR, Hrdina R, Kagan HB, Kočovský P, Kochetkov KA, Larionov OV, Lyssenko KA, North M, Polášek M, Peregudov AS, Prisyazhnyuk VV, Vyskoil S (2003) Synthesis of α-amino acids via asymmetric phase transfer-catalyzed alkylation of achiral nickel(II) complexes of glycine-derived schiff bases. J Am Chem Soc 125:12860–12871
Belokon YN, Harutyunyan S, Vorontsov EV, Peregudov AS, Chrustalev VN, Kochetkov KA, Pripadchev D, Sagyan AS, Beck AK, Seebach D (2004) Nucleophilic addition to an achiral dehydroalanine Schiff base Ni(II) complex as a route to amino acids. A case of stereodetermining asymmetric protonation in the presence of TADDOL. ARKIVOC iii:132–150
Belokon YN, Gugkaeva ZT, Maleev VI, Moskalenko MA, Tsaloev AT, Khrustalev VN, Hakobyan KV (2011) Four hydroxyls are better than two. The use of a chiral lithium salt of 3, 3′-bis-methanol-2, 2′-binaphthol as a multifunctional catalyst of enantioselective Michael addition reactions. Tetrahedron Asymmetr 22:167–172
Cativiela C, de Díaz Villegas MD (1998) Stereoselective synthesis of quaternary α-amino acids. Part 1. Acyclic compounds. Tetrahedron Asymmetr 9:3517–3599
Cheon CH, Yamamoto H (2008) A brønsted acid catalyst for the enantioselective protonation reaction. J Am Chem Soc 130:9246–9247
Corey EJ, Noe MC, Xu F (1998) Highly enantioselective synthesis of cyclic and functionalized α-amino acids by means of a chiral phase transfer catalyst. Tetrahedron Lett 39:5347–5350
Duthaler RO (1994) Recent developments in the stereoselective synthesis of α-aminoacids. Tetrahedron 50:1539–1650
Emori E, Arai T, Sasai H, Shibasaki M (1998) A catalytic michael addition of thiols to α, β-unsaturated carbonyl compounds: asymmetric michael additions and asymmetric protonations. J Am Chem Soc 120:4043–4044
Fehr C (1996) Enantioselective protonation of enolates and enols. Angew Chem Int Ed Engl 35:2566–2587
Huffman CW, Scelly WG (1963) Glutamic acid: chemical syntheses and resolutions. Chem Rev 63:625–644
Kirshner S, Ahmad N, Magnell K (1968) Optical rotatory dispersion and the Pfeiffer effect in coordination compounds. Coord Chem Rev 3:201–206
Kobayashi S, Yamashita Y (2011) Alkaline Earth metal catalysts for asymmetric reactions. Acc Chem Res 44:58–71
Kumar A, Salunkhe RV, Rane RA, Dike SY (1991) Novel catalytic enantioselective protonation (proton transfer) in Michael addition of benzenethiol to α-acrylacrylates: synthesis of (S)-naproxen and α-arylpropionic acids or esters. J Chem Soc Chem Commun 485–486
Leow D, Lin S, Chittimalla SK, Fu X, Tan CH (2008) Enantioselective protonation catalyzed by a chiral bicyclic guanidine derivative. Angew Chem Int Ed Engl 47:5641–5645
Lygo B, Crosby J, Lowdon TR, Peterson JA, Wainwright PG (2001) Studies on the enantioselective synthesis of α-amino acids via asymmetric phase-transfer catalysis. Tetrahedron 57:2403–2409
Ma J-A (2003) Recent developments in the catalytic asymmetric synthesis of α-and β-amino acids. Angew Chem Int Ed Engl 42:4290–4299
Maruoka K, Ooi T (2003) Enantioselective amino acid synthesis by chiral phase-transfer catalysis. Chem Rev 103:3013–3028
Muñoz-Muñiz O, Juaristi E (2003) Enantioselective protonation of prochiral enolates in the asymmetric synthesis of (S)-naproxen. Tetrahedron Lett 44:2023–2026
Nájera C, Sansano JM (2007) Catalytic asymmetric synthesis of α-amino acids. Chem Rev 107:4584–4671
Navarre L, Martinez R, Genet JP, Darses S (2008) Access to enantioenriched α-amino esters via Rhodium-catalyzed 1, 4-addition/enantioselective protonation. J Am Chem Soc 130:6159–6169
Nishimura K, Ono M, Nagaoka Y, Tomioka K (2001) Catalytic Enantioselective protonation of Lithium ester enolates generated by conjugate addition of arylthiolate to enoates. Angew Chem Int Ed Engl 40:440–442
O’Donnell MJ, Bennett WD, Bruder WA, Jacobsen WN, Knuth K, LeClef B, Polt RL, Bordwell FG, Mrozack SR, Cripe TR (1988) Acidities of glycine Schiff bases and alkylation of their conjugate bases. J Am Chem Soc 110:8520–8525
Pfeiffer P, Quell K (1931) Über einen neuen Effekt in Lösungen optisch-aktiver Substanzen. Ber 64: 2667–2671
Pracejus H, Wilcke FW, Hanemann K (1977) Asymmetrisch katalysierte Additionen von Thiolen an α-Aminoacrylsäure-Derivate und Nitroolefine. J Prakt Chem 319:219–229
Smith DJ, Yap GPA, Kelley JA, Schneider JP (2011) Enhanced stereoselectivity of a Cu(II) complex chiral auxiliary in the synthesis of Fmoc-l-γ-carboxyglutamic acid. J Org Chem 76:1513–1520
Tsubogo T, Kano Y, Ikemoto K, Yamashita Y, Kobayashi S (2010) Synthesis of optically active, unnatural α-substituted glutamic acid derivatives by a chiral calcium-catalyzed 1, 4-addition reaction. Tetrahedron Asymmetr 21:1221–1225
Vyskočil S, Meca L, Tišlerová I, Císařová I, Polášek M, Harutyunyan SR, Belokon YN, Stead RMJ, Farrugia L, Lockhart SC, Mitchell WL, Kočovský P (2002) 2, 8′-Disubstituted-1, 1′-binaphthyls: a new pattern in chiral ligands. Chem Eur J 8:4633–4648
Wang Q, Chen X, Tao L, Wang L, Xiao D, Yu XQ, Pu L (2007) Enantioselective fluorescent recognition of amino alcohols by a chiral tetrahydroxyl 1,1′-binaphthyl compound. J Org Chem 97–101
Williams RM (1989) Synthesis of optically active α-amino acids. Pergamon Press, Oxford
Yanagisawa A, Yamamoto H (1999) Protonation of Enolates. In: Jacobsen EN, Pflatz A, Yamamoto H (eds) Comprhensive asymmetric catalysis. Springer, Heidelberg, pp 1295–1306
Yanagisawa A, Watanabe T, Kikuchi T, Yamamoto H (2000) Catalytic enantioselective protonation of Lithium enolates with Chiral imides. J Org Chem 65:2979–2983
Acknowledgments
The support of this work by RFBR Grants No. 11-03-00206-a and 09-03-00730 is gratefully acknowledged. The authors thank Prof. K. A. Lyssenko (A. N. Nesmeyanov Institute of Organoelement Compounds RAS) for performing the X-ray structure analysis of compound 2 and Dr. M. G. Ezernitskaya for IR studies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Experimental data available free of charge in the supplementary section.
Rights and permissions
About this article
Cite this article
Belokon, Y.N., Gugkaeva, Z.T., Hakobyan, K.V. et al. Using the same organocatalyst for asymmetric synthesis of both enantiomers of glutamic acid-derived Ni(II) complexes via 1,4-additions of achiral glycine and dehydroalanine Schiff base Ni(II) complexes. Amino Acids 43, 299–308 (2012). https://doi.org/10.1007/s00726-011-1076-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1076-y