Abstract
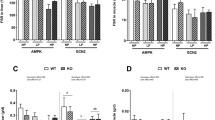
The purpose of this work was to examine whether changes in dietary protein levels could elicit differential responses of tissue proteolysis and the pathway involved in this response. In rats fed with a high protein diet (55%) for 14 days, the liver was the main organ where adaptations occurred, characterized by an increased protein pool and a strong, meal-induced inhibition of the protein breakdown rate when compared to the normal protein diet (14%). This was associated with a decrease in the key-proteins involved in expression of the ubiquitin–proteasome and autophagy pathway gene and a reduction in the level of hepatic ubiquitinated protein. In hepatocytes, we demonstrated that the increase in amino acid (AA) levels was sufficient to down-regulate the ubiquitin proteasome pathway, but this inhibition was more potent in the presence of insulin. Interestingly, AICAR, an adenosine monophosphate-activated protein kinase (AMPK) activator, reversed the inhibition of protein ubiquination induced by insulin at high AA concentrations. Rapamycin, an mammalian target of rapamycin (mTOR) inhibitor, reversed the inhibition of protein ubiquination induced by a rise in insulin levels with both high and low AA concentrations. Moreover, in both low and high AA concentrations in the presence of insulin, AICAR decreased the mTOR phosphorylation, and in the presence of both AICAR and rapamycin, AICAR reversed the effects of rapamycin. These results demonstrate that the inhibition of AMPK and the activation of mTOR transduction pathways, are required for the down-regulation of protein ubiquitination in response to high amino acid and insulin concentrations.






Similar content being viewed by others
References
Attaix D, Aurousseau E, Combaret L, Kee A, Larbaud D et al (1998) Ubiquitin–proteasome-dependent proteolysis in skeletal muscle. Reprod Nutr Dev 38(2):153–165
Attaix D, Combaret L, Pouch MN, Taillandier D (2001) Regulation of proteolysis. Curr Opin Clin Nutr Metab Care 4(1):45–49
Azzout-Marniche D, Gaudichon C, Blouet C, Bos C, Mathe V et al (2007) Liver glyconeogenesis: a pathway to cope with postprandial amino acid excess in high-protein fed rats? Am J Physiol Regul Integr Comp Physiol 292(4):R1400–R1407
Balage M, Sinaud S, Prod'homme M, Dardevet D, Vary TC et al (2001) Amino acids and insulin are both required to regulate assembly of the eIF4E·eIF4G complex in rat skeletal muscle. Am J Physiol Endocrinol Metab 281(3):E565–E574
Baum JI, Layman DK, Freund GG, Rahn KA, Nakamura MT et al (2006) A reduced carbohydrate, increased protein diet stabilizes glycemic control and minimizes adipose tissue glucose disposal in rats. J Nutr 136(7):1855–1861
Bleiberg-Daniel F, Lamri Y, Feldmann G, Lardeux B (1994) Glucagon administration in vivo stimulates hepatic RNA and protein breakdown in fed and fasted rats. Biochem J 299(Pt 3):645–649
Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ (1995) Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem 270(5):2320–2326
Blommaart EF, Luiken JJ, Meijer AJ (1997) Autophagic proteolysis: control and specificity. Histochem J 29(5):365–385
Blouet C, Mariotti F, Azzout-Marniche D, Bos C, Mathe V et al (2006) The reduced energy intake of rats fed a high-protein low-carbohydrate diet explains the lower fat deposition, but macronutrient substitution accounts for the improved glycemic control. J Nutr 136(7):1849–1854
Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL et al (1997) Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 94(26):14930–14935
Busquets S, Alvarez B, Lopez-Soriano FJ, Argiles JM (2002) Branched-chain amino acids: a role in skeletal muscle proteolysis in catabolic states? J Cell Physiol 191(3):283–289
Capel F, Prod’homme M, Bechet D, Taillandier D, Balage M et al (2008) Lysosomal and proteasome-dependent proteolysis are differentially regulated by insulin and/or amino acids following feeding in young, mature and old rats. J Nutr Biochem 20(8):570–576
Cheng SW, Fryer LG, Carling D, Shepherd PR (2004) Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem 279(16):15719–15722 Epub 12004 Feb 15717
Chevalier L, Bos C, Gryson C, Luengo C, Walrand S et al (2009) High-protein diets differentially modulate protein content and protein synthesis in visceral and peripheral tissues in rats. Nutrition 25(9):932–939
Chotechuang N, Azzout-Marniche D, Bos C, Chaumontet C, Gausseres N et al (2009) mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab 297(6):E1313–E1323
Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D et al (2005) A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J Physiol 569(Pt 2):489–499
Costelli P, Reffo P, Penna F, Autelli R, Bonelli G et al (2005) Ca2+-dependent proteolysis in muscle wasting. Int J Biochem Cell Biol 37(10):2134–2146
Del Roso A, Vittorini S, Cavallini G, Donati A, Gori Z et al (2003) Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp Gerontol 38(5):519–527
Demarchi F, Bertoli C, Copetti T, Tanida I, Brancolini C et al (2006) Calpain is required for macroautophagy in mammalian cells. J Cell Biol 175(4):595–605
Ding X, Price SR, Bailey JL, Mitch WE (1997) Cellular mechanisms controlling protein degradation in catabolic states. Miner Electrolyte Metab 23(3–6):194–197
Elsasser S, Finley D (2005) Delivery of ubiquitinated substrates to protein-unfolding machines. Nat Cell Biol 7(8):742–749
Flakoll PJ, Kulaylat M, Frexes-Steed M, Hourani H, Brown LL et al (1989) Amino acids augment insulin's suppression of whole body proteolysis. Am J Physiol 257(6 Pt 1):E839–E847
Forslund AH, Hambraeus L, Olsson RM, El-Khoury AE, Yu YM et al (1998) The 24-h whole body leucine and urea kinetics at normal and high protein intakes with exercise in healthy adults. Am J Physiol 275(2 Pt 1):310–320
Gelfand RA, Barrett EJ (1987) Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest 80(1):1–6
Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol Rev 83(3):731–801
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A et al (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30(2):214–226
Hamel FG, Upward JL, Siford GL, Duckworth WC (2003) Inhibition of proteasome activity by selected amino acids. Metabolism 52(7):810–814
Hamel FG, Fawcett J, Bennett RG, Duckworth WC (2004) Control of proteolysis: hormones, nutrients, and the changing role of the proteasome. Curr Opin Clin Nutr Metab Care 7(3):255–258
Harber MP, Schenk S, Barkan AL, Horowitz JF (2005) Effects of dietary carbohydrate restriction with high protein intake on protein metabolism and the somatotropic axis. J Clin Endocrinol Metab 90(9):5175–5181
Hicke L, Dunn R (2003) Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 19:141–172
Ilian MA, Forsberg NE (1992) Gene expression of calpains and their specific endogenous inhibitor, calpastatin, in skeletal muscle of fed and fasted rabbits. Biochem J 287(Pt 1):163–171
Kadowaki M, Kanazawa T (2003) Amino acids as regulators of proteolysis. J Nutr 133(6 Suppl 1):2052–2056
Kadowaki M, Poso AR, Mortimore GE (1992) Parallel control of hepatic proteolysis by phenylalanine and phenylpyruvate through independent inhibitory sites at the plasma membrane. J Biol Chem 267(31):22060–22065
Kanazawa T, Taneike I, Akaishi R, Yoshizawa F, Furuya N et al (2004) Amino acids and insulin control autophagic proteolysis through different signaling pathways in relation to mTOR in isolated rat hepatocytes. J Biol Chem 279(9):8452–8459
Kettelhut IC, Wing SS, Goldberg AL (1988) Endocrine regulation of protein breakdown in skeletal muscle. Diabetes Metab Rev 4(8):751–772
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721
Lacroix M, Gaudichon C, Martin A, Morens C, Mathe V et al (2004) A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol 287(4):R934–R942
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6(4):463–477
Lusk G (1919) The elements of the science of Nutrition. W. B. Saunders Company, Philadelphia
Marinovic AC, Zheng B, Mitch WE, Price SR (2007) Tissue-specific regulation of ubiquitin (UbC) transcription by glucocorticoids: in vivo and in vitro analyses. Am J Physiol Renal Physiol 292(2):F660–F666
Medina R, Wing SS, Haas A, Goldberg AL (1991) Activation of the ubiquitin-ATP-dependent proteolytic system in skeletal muscle during fasting and denervation atrophy. Biomed Biochim Acta 50(4–6):347–356
Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT et al (2006) AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem 281(46):34870–34879
Miotto G, Venerando R, Khurana KK, Siliprandi N, Mortimore GE (1992) Control of hepatic proteolysis by leucine and isovaleryl-L-carnitine through a common locus. Evidence for a possible mechanism of recognition at the plasma membrane. J Biol Chem 267(31):22066–22072
Mitch WE, Goldberg AL (1996) Mechanisms of muscle wasting. The role of the ubiquitin–proteasome pathway. N Engl J Med 335(25):1897–1905
Mortimore GE, Mondon CE (1970) Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem 245(9):2375–2383
Mortimore GE, Hutson NJ, Surmacz CA (1983) Quantitative correlation between proteolysis and macro- and microautophagy in mouse hepatocytes during starvation and refeeding. Proc Natl Acad Sci USA 80(8):2179–2183
Mortimore GE, Poso AR, Lardeux BR (1989) Mechanism and regulation of protein degradation in liver. Diabetes Metab Rev 5(1):49–70
Mounier C, Posner BI (2006) Transcriptional regulation by insulin: from the receptor to the gene. Can J Physiol Pharmacol 84(7):713–724
Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J (1995) Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J Clin Invest 95(6):2926–2937
Otani K, Han DH, Ford EL, Garcia-Roves PM, Ye H et al (2004) Calpain system regulates muscle mass and glucose transporter GLUT4 turnover. J Biol Chem 279(20):20915–20920
Pacy PJ, Price GM, Halliday D, Quevedo MR, Millward DJ (1994) Nitrogen homeostasis in man: the diurnal responses of protein synthesis and degradation and amino acid oxidation to diets with increasing protein intakes. Clin Sci (Lond) 86(1):103–116
Poso AR, Wert JJ Jr, Mortimore GE (1982) Multifunctional control of amino acids of deprivation-induced proteolysis in liver. Role of leucine. J Biol Chem 257(20):12114–12120
Price GM, Halliday D, Pacy PJ, Quevedo MR, Millward DJ (1994) Nitrogen homeostasis in man: influence of protein intake on the amplitude of diurnal cycling of body nitrogen. Clin Sci (Lond) 86(1):91–102
Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC et al (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320(5882):1496–1501
Schworer CM, Mortimore GE (1979) Glucagon-induced autophagy and proteolysis in rat liver: mediation by selective deprivation of intracellular amino acids. Proc Natl Acad Sci USA 76(7):3169–3173
Seglen PO, Gordon PB, Poli A (1980) Amino acid inhibition of the autophagic/lysosomal pathway of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta 630(1):103–118
Shaw RJ (2008) mTOR signaling: RAG GTPases transmit the amino acid signal. Trends Biochem Sci 33(12):565–568
Stepien M, Gaudichon C, Azzout-Marniche D, Fromentin G, Tome D et al (2010) Postprandial nutrient partitioning but not energy expenditure is modified in growing rats during adaptation to a high-protein diet. J Nutr 140(5):939–945
Thivierge MC, Bush JA, Suryawan A, Nguyen HV, Orellana RA et al (2005) Whole-body and hindlimb protein breakdown are differentially altered by feeding in neonatal piglets. J Nutr 135(6):1430–1437
Tischler ME, Desautels M, Goldberg AL (1982) Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem 257(4):1613–1621
Tong JF, Yan X, Zhu MJ, Du M (2009) AMP-activated protein kinase enhances the expression of muscle-specific ubiquitin ligases despite its activation of IGF-1/Akt signaling in C2C12 myotubes. J Cell Biochem 108(2):458–468
Waterlow JC (2006) The effects of food and hormones on protein turnover in the whole body and regions. Cabi Publishing, Wallingford, pp 120–141
Wing SS, Banville D (1994) 14 kDa ubiquitin-conjugating enzyme: structure of the rat gene and regulation upon fasting and by insulin. Am J Physiol 267(1 Pt 1):39–48
Wing SS, Goldberg AL (1993) Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am J Physiol 264(4 Pt 1):668–676
Wing SS, Haas AL, Goldberg AL (1995) Increase in ubiquitin-protein conjugates concomitant with the increase in proteolysis in rat skeletal muscle during starvation and atrophy denervation. Biochem J 307(Pt 3):639–645
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chotechuang, N., Azzout-Marniche, D., Bos, C. et al. Down-regulation of the ubiquitin–proteasome proteolysis system by amino acids and insulin involves the adenosine monophosphate-activated protein kinase and mammalian target of rapamycin pathways in rat hepatocytes. Amino Acids 41, 457–468 (2011). https://doi.org/10.1007/s00726-010-0765-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0765-2