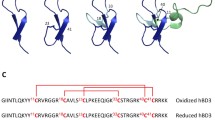
Abstract
Human beta-defensins are 2–5 kDa, cationic, microbicidal peptides, which represent the first-line host defense against several Gram-negative and Gram-positive bacteria, fungi and viruses. They contain a conserved disulfide-bridge pattern of three pairs of intramolecular cystine bonds. The well-known public health problem related with the growing number of multiresistant bacteria has driven research to look for novel antibiotics, such beta-defensins and a feasible way to produce them. Heterologous expression of beta-defensins could be one way to generate large quantities of beta-defensins for clinical research; however, heterologous expression of beta-defensins has some biochemical problems, such toxicity toward the host cell, peptide degradation by proteolytic cell enzymes, size, folding constrains and low recombinant peptide yields. In this communication, several heterologous systems for producing human beta-defensins are reviewed.

Similar content being viewed by others
References
Aerts AM, Thevissen K, Bresseleers SM, Sels J, Wouters P, Cammue BPA, François IEJA (2007) Arabidopsis thaliana plants expressing human-defensin-2 are more resistant to fungal attack: functional homology between plant and human defensins. Plant Cell Rep 26:1391–1398. doi:10.1007/s00299-007-0329-4
Bass SH, Yansura DG (2000) Application of the E. coli trp promoter. J Mol Biol Res Protoc Rev Appl 16:253–260. doi:10.1385/0-89603-480-1:55
Bensch KW, Raida M, Mägert H-J, Schulz-Knappe P, Forssmann W-G (1995) hBD-I: a novel-defensin from human plasma. FEBS 368:331–335. doi:10.1016/0014-5793(95)00687-5
Chen H, Xu Z, Xu N, Cen P (2005) Efficient production of a soluble fusion protein containing human-defensin-2 in E. coli cell-free system. J Biotechnol 115:307–315. doi:10.1016/j.jbiotec.2004.08.012
Chen H, Xu Z, Xu N, Cen P (2006) High-level expression of human-defensin-2 gene with rare codons in E. coli cell-free system. Prot Pept Lett 13:155–161. doi:10.2174/092986606775101724
Chen H, Fan L, Xu Z, Yin X, Cen P (2007) Efficient production of soluble human-defensin-3–4 fusion proteins in Escherichia coli cell-free system. Process Biochem 42:423–428. doi:10.1016/j.procbio.2006.10.002
Cipáková I, Hostinová E (2005) Production of the human-defensin using Saccharomyces cerevisiae as a host. Prot Pept Lett 12:551–554. doi:10.2174/0929866054395761
Cipáková I, Hostinová E, Gasperík J, Velebny V (2004) High-level expression and purification of a recombinant hBD-1Fused to LMM protein in Escherichia coli. Prot Expr Purif 37:207–212. doi:10.1016/j.pep.2004.04.024
Dhople V, Krukemeyer A, Ramamoorthy A (2006) The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim Biophys Acta 1758:1499–1512. doi:10.1016/j.bbamem.2006.07.007
Estrada G, Garcia BI, Schiavon E, Ortiz E, Cestele S, Wanke E, Possani LD, Corzo G (2007) Four disulfide-bridged scorpion beta neurotoxin CssII: heterologous expression and proper folding in vitro. Biochim Biophys Acta 1770:1161–1168. doi:10.1016/j.bbagen.2007.04.006
Fang X, Peng L, Xu Z, Wu J, Cen P (2002) Cloning and expression of human-defensin-2 gene in Escherichia coli. Prot Pept Lett 9:31–37. doi:10.2174/0929866023409011
Fleming A (1922) On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc Lond 93:306–317. doi:10.1098/rspb.1922.0023
Ganz T (2002) Versatile defensins. Science 298:977–978. doi:10.1126/science.1078708
Ganz T (2003) The role of antimicrobial peptides in innate immunity. Integr Comp Biol 43:300–304. doi:10.1093/icb/43.2.300
Hancock REW (2001) Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis 1:156–164. doi:10.1016/S1473-3099(01)00092-5
Hancock REW, Chapple DS (1999) Peptide antibiotics. Antimicrob Agents Chemother 43:1317–1323
Hancock REW, Diamond G (2000) The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 8:402–410. doi:10.1016/S0966-842X(00)01823-0
Harder J, Bartels J, Christophers E, Schröder J-M (1997) A peptide antibiotic from human Skin. Nature 387:861. doi:10.1038/43088
Harder J, Bartels J, Christophers E, Schröder J-M (2001) Isolation and characterization of human-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 276:5707–5713. doi:10.1074/jbc.M008557200
Hoover DM, Rajashankar KR, Blumenthali R, Purii Anu, Oppenheim JJ, Chertov O, Lubkowski J (2000) The structure of human-defensin-2 shows evidence of higher order oligomerization. J Biol Chem 275:32911–32918. doi:10.1074/jbc.M006098200
Hoover DM, Chertov O, Lubkowski J (2001) The structure of human-defensin-1. J Biol Chem 276:39021–39026. doi:10.1074/jbc.M103830200
Hoover DM, Wu Z, Tucker K, Lu W, Lubkowski J (2003) Antimicrobial characterization of human-defensin 3 derivatives. Antimicrob Agents Chemother 47:2804–2809. doi:10.1128/AAC.47.9.2804-2809.2003
Huang L, Wang J, Zhong Z, Peng L, Chen H, Xu Z, Cen P (2006) Production of bioactive human-defensin-3 in Escherichia coli by soluble fusion expression. Biotechnol Lett 28:627–632. doi:10.1007/s10529-006-0024-5
Huang L, Ching CB, Jiang R, Leong SSJ (2008) Production of bioactive human-defensin 5 and 6 in Escherichia coli by soluble fusion expression. Prot Exp Purif 61:168–174. doi:10.1016/j.pep.2008.05.016
Huang L, Leong SSJ, Jiang R (2009) Soluble fusion expression and characterization of bioactive human-defensin 26 and 27. Appl Microbiol Biotechnol 84:301–308. doi:10.1007/s00253-009-1982-z
Lehmann J, et al. (2002) Expression of human β-defensins 1 and 2 in kidneys with chronic bacterial infection. BMC Infect Dis 20. doi:10.1186/1471-2334-2-20
Lehrer RI (2004) Primate defensins. Nat Rev Microbiol 2:727–737. doi:10.1038/nrmicro976
Lehrer RI, Ganz T (2002) Defensins of vertebrate animals. Curr Opin Immunol 14:96–102. doi:10.1016/S0952-7915(01)00303-X
Li X, Gasic K, Cammue B, Broekaert W, Korban SS (2003) Transgenic rose lines harboring an antimicrobial protein gene, Ace-AMP1, demonstrate enhanced resistance to powdery Mildew (Sphaerotheca pannosa). Planta 218:226–232. doi:10.1007/s00425-003-1093-5
Li-gang S, Xi-cheng L, You-yong L, Gen-yu W, Wen-mei L (2007) Soluble expression of active human-defensin-3 in Escherichia coli and its effects on the growth of host cells. Chin Med J 120:708–713
Mathews M, Jia HP, Guthmiller JM, Losh G, Graham S, Johnson GK, Tack BF, McCray PB (1999) Production of-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun 67:2740–2745
Peng L, Xu Z, Fang X, Wang F, Cen P (2004a) High-level expression of soluble human-defensin-2 in Escherichia coli. Process Biochem 39:2199–2205. doi:10.1016/j.procbio.2003.11.011
Peng L, Xu Z, Fang X, Wang F, Yang S, Cen P (2004b) Preferential codons enhancing the expression level of human-defensin-2 in recombinant Escherichia coli. Prot Pept Lett 11:339–344. doi:10.2174/0929866043406760
Piers KL, Brown MH, Hancock REW (1993) Recombinant DNA procedures for producing small antimicrobial cationic peptides in bacteria. Gene 134:7–13. doi:10.1016/0378-1119(93)90168-3
Porter EM, Ev Dam, Valore EV, Ganz T (1997) Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun 65:2396–2401
Schibli DJ, Hunter HN, Aseyev V, Starner TD, Wiencek JM, McCray PB, Tack BF, Vogel HJ (2002) The solution structures of the human-defensins lead to a better understanding of the potent bactericidal activity of HBD3 against Staphylococcus aureus. J Biol Chem 277:8279–8289. doi:10.1074/jbc.M108830200
Schneider JJ, Unholzer A, Schaller M, Schäfer-Korting M, Korting HC (2005) Human Defensins. J Mol Med 83:587–595. doi:10.1007/s00109-005-0657-1
Schöder J-M, Harder J (1999) Human-defensins-2. Int J Biochem Cell Biol 31:645–651. doi:10.1016/S1357-2725(99)00013-8
Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, Welsh MJ, Casavant TL, Paul B, McCray J (2002) Discovery of five conserved-defensin gene clusters using a computational search strategy. PNAS 99:2129–2133. doi:10.1073/pnas.042692699
Schwaab M, Hansen S, Pearson MD, Shagdarsuren S, Dazert S (2009) Human-defensins—at the front line of the peritonsillar abscess. Eur J Clin Microbiol Infect Dis. doi:10.1007/s10096-008-0695-z
Song W, Shi Y, Xiao M, Lu H, Qu T, Li P, GangWu TianY (2009) In vitro bactericidal activity of recombinant human-defensin-3 against pathogenic bacterial strains in human tooth root canal. Int J Antimicrob Agents 33:237–243. doi:10.1016/j.ijantimicag.2008.05.022
Terpe K (2006) Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to Commercial Systems. Appl Microbiol Biotechnol 72:211–222. doi:10.1007/s00253-006-0465-8
Valore EV, Ganz T (1997) Antibacterial peptide protocols. In: W-M Shafer (ed) Methods in molecular biology (1 edn). Humana Press Inc., Tokowa, NJ, USA
Valore EV, Park CH, Quayle AJ, Wiles KR, Paul B, McCray J, Ganz T (1998) Human-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest 101:1633–1642. doi:10.1172/JCI1861
Vargues T et al (2009) Efficient production of human-defensin 2 (HBD2) in Escherichia coli. Prot Pept Lett 16:668–676. doi:10.2174/092986609788490122
Wang F, Fang X, Xu Z, Peng L, Cen P (2004) Fusion expression of human-defensin-2 from multiple joined genes in Escherichia coli. Prep Biochem Biotechnol 34:215–225. doi:10.1081/PB-200026797
Wei Q, Kim YS, Seo JH, Jang WS, Lee IH, Cha HJ (2005) Facilitation of expression and purification of an antimicrobial peptide by fusion with baculoviral polyhedrin in Escherichia coli. Appl Environ Microbiol 71. doi:10.1128/AEM.71.9.5038-5043.2005
Wong JH, Xia L, Ng TB (2007) A review of defensins of diverse origins. Curr Prot Peptide Sci 8:446–459. doi:10.2174/138920307782411446
Wu Z, Hoover DM, Yang D, Boulègue C, Santamaria F, Oppenheim JJ, Lubkowski J, Lu W (2003) Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human-defensin 3. PNAS 100:8880–8885. doi:10.1073/pnas.1533186100
Xu Z, Peng L, Zhong Z, Fang X, Cen P (2006a) High-level expression of a soluble functional antimicrobial peptide, human-defensin 2, in Escherichia coli. Biotechnol Prog 22:382–386. doi:10.1021/bp0502680
Xu Z, Zhong Z, Huang L, Peng L, Wang F, Cen P (2006b) High-level production of bioactive human-defensin-4 in Escherichia coli by soluble fusion expression. Appl Microbiol Biotechnol 72:471–479. doi:10.1007/s00253-005-0287-0
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395. doi:10.1038/415389a
Acknowledgments
This work was supported by grants from the Dirección General de Asuntos del Personal Académico (DGAPA-UNAM) IN220809 to GC. Ligia Luz Corrales is recipient of a Ph.D. scholarship (331398/229367) from CONACyT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corrales-Garcia, L.L., Possani, L.D. & Corzo, G. Expression systems of human β-defensins: vectors, purification and biological activities. Amino Acids 40, 5–13 (2011). https://doi.org/10.1007/s00726-010-0493-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0493-7