Abstract
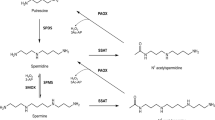
N-alkylated polyamine analogues have potential as anticancer and antiparasitic drugs. However, their metabolism in the host has remained incompletely defined thus potentially limiting their utility. Here, we have studied the degradation of three different spermine analogues N,N′-bis-(3-ethylaminopropyl)butane-1,4-diamine (DESPM), N-(3-benzyl-aminopropyl)-N′-(3-ethylaminopropyl)butane-1,4-diamine (BnEtSPM) and N,N′-bis-(3-benzylaminopropyl)butane-1,4-diamine (DBSPM) and related mono-alkylated derivatives as substrates of recombinant human polyamine oxidase (APAO) and spermine oxidase (SMO). APAO and SMO metabolized DESPM to EtSPD [Km(APAO) = 10 μM, kcat(APAO) = 1.1 s−1 and Km(SMO) = 28 μM, kcat(SMO) = 0.8 s−1, respectively], metabolized BnEtSPM to EtSPD [Km(APAO) = 0.9 μM, kcat(APAO) = 1.1 s−1 and Km(SMO) = 51 μM, kcat(SMO) = 0.4 s−1, respectively], and metabolized DBSPM to BnSPD [Km(APAO) = 5.4 μM, kcat(APAO) = 2.0 s−1 and Km(SMO) = 33 μM, kcat(SMO) = 0.3 s−1, respectively]. Interestingly, mono-alkylated spermine derivatives were metabolized by APAO and SMO to SPD [EtSPM Km(APAO) = 16 μM, kcat(APAO) = 1.5 s−1; Km(SMO) = 25 μM, kcat(SMO) = 8.2 s−1; BnSPM Km(APAO) = 6.0 μM, kcat(APAO) = 2.8 s−1; Km(SMO) = 19 μM, kcat(SMO) = 0.8 s−1, respectively]. Surprisingly, EtSPD [Km(APAO) = 37 μM, kcat(APAO) = 0.1 s−1; Km(SMO) = 48 μM, kcat(SMO) = 0.05 s−1] and BnSPD [Km(APAO) = 2.5 μM, kcat(APAO) = 3.5 s−1; Km(SMO) = 60 μM, kcat(SMO) = 0.54 s−1] were metabolized to SPD by both the oxidases. Furthermore, we studied the degradation of DESPM, BnEtSPM or DBSPM in the DU145 prostate carcinoma cell line. The same major metabolites EtSPD and/or BnSPD were detected both in the culture medium and intracellularly after 48 h of culture. Moreover, EtSPM and BnSPM were detected from cell samples. Present data shows that inducible SMO parallel with APAO could play an important role in polyamine based drug action, i.e. degradation of parent drug and its metabolites, having significant impact on efficiency of these drugs, and hence for the development of novel N-alkylated polyamine analogues.



Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- APAO:
-
(Acetyl)polyamine oxidase (exo-N4-amino) [EC 1.5.3.11]
- BnDAP:
-
N1-benzyl-propane-1,3-diamine
- BnDAP-4D:
-
N1-benzyl-2,2,3,3-2H4-propane-1,3-diamine
- BnEtSPM:
-
N-(3-benzylaminopropyl)-N′-(3-ethylaminopropyl)butane-1,4-diamine
- BnEtSPM-8D:
-
N-(3-benzylamino-1,1,2,2-2H4-propyl)-N′-(3-ethylamino-1,1,2,2-2H4-propyl)butane-1,4-diamine
- BnNH2 :
-
Benzylamine
- BnNH2-2D:
-
α,α-2H2-benzylamine
- BnSPD:
-
N1-(3-benzylaminopropyl)butane-1,4-diamine
- BnSPD-4D:
-
N1-(3-benzylamino-1,1,2,2-2H4-propyl)butane-1,4-diamine
- BnSPM:
-
N-(3-aminopropyl)-N′-(3-benzylaminopropyl)butane-1,4-diamine
- BnSPM-4D:
-
N-(3-aminopropyl)-N′-(3-benzylamino-1,1,2,2-2H4-propyl)butane-1,4-diamine
- CID:
-
Collision energy
- DAP:
-
Propane-1,3-diamine
- DAP-2D:
-
1,1-2H2-propane-1,3-diamine
- DBSPM:
-
N,N′-bis-(3-benzylaminopropyl)butane-1,4-diamine
- DBSPM-8D:
-
N,N′-bis-(3-benzylamino-1,1,2,2-2H4-propyl)butane-1,4-diamine
- DENSPM:
-
Diethylnorspermine, N,N′-bis-(3-ethylaminopropyl)-propane-1,3-diamine
- DESPM:
-
N,N′-bis-(3-ethylaminopropyl)butane-1,4-diamine
- DESPM-4D:
-
N,N′-bis-(3-ethylamino-1,1-2H2-propyl)butane-1,4-diamine
- EtDAP:
-
N1-ethylpropane-1,3-diamine
- EtDAP-2D:
-
N1-ethyl-3,3-2H2-propane-1,3-diamine
- EtSPD:
-
N1-(3-ethylaminopropyl)butane-1,4-diamine trihydrochloride
- EtSPD-2D:
-
N1-(3-ethylamino-1,1-2H2-propyl)butane-1,4-diamine
- EtSPM:
-
N-(3-aminopropyl)-N′-(3-ethylaminopropyl)butane-1,4-diamine
- EtSPM-2D:
-
N-(3-aminopropyl)-N′-(3-ethylamino-1,1-2H2-propyl)butane-1,4-diamine
- FDA:
-
US, Food and Drug Administration, USA
- HFBA:
-
Heptafluorobutyric acid
- HPLC:
-
High pressure liquid chromatography
- IS:
-
Internal standard
- LC-MS/MS:
-
Liquid chromatography-electrospray ionization-tandem mass spectrometry
- MDL 72527:
-
N,N′-bis-(2,3-butadienyl)-1,4-butanediamine
- NMR:
-
Nuclear magnetic resonance
- N1-AcSPD:
-
N1,N12-diacetylspermine
- N1-AcSPM:
-
N1,N12-diacetylspermidine
- N1,N12-DiAcSPM:
-
N1,N12-Diacetylspermine
- PA:
-
Polyamine
- PUT:
-
Putrescine, butane-1,4-diamine
- PUT-8D:
-
1,1,2,2,3,3,4,4-2H8-butane-1,4-diamine
- SMO:
-
(PAOh1), Spermine oxidase [EC 1.5.3.-]
- SPD:
-
Spermidine, N 1-(3-aminopropyl)butane-1,4-diamine
- SPD-2D:
-
N1-(3-amino-1,1-2H2-propyl)butane-1,4-diamine
- SPM:
-
Spermine, N,N′-bis-(3-aminopropyl)butane-1,4-diamine
- SPM-4D:
-
N-(3-amino-1,1,2,2-2H4-propyl)-N′-(3-aminopropyl)butane-1,4-diamine
- SRM:
-
Selected reaction monitoring
- SSAT:
-
Spermidine/spermine N 1-acetyltransferase [EC 2.3.1.57]
- STD:
-
Standard
- QC:
-
Quality control
- QL:
-
Qualifier ion
- QT:
-
Quantifier ion
References
Agostinelli E, Arancia G, Dalla Vedova I, Belli F, Marra M, Salvi M, Toninello A (2004) The biological functions of polyamine oxidation products by amine oxidases: perspectives of clinical applications. Amino Acids 3–4:347–358
Bacchi CJ, Yarlett N, Faciane E, Bi X, Rattendi D, Weiss LM, Woster PM (2009) Metabolism of an alkyl polyamine analog by a polyamine oxidase from the microsporidian Encephalitozoon cuniculi. Antimicrob Agents Chemother 53:2599–2604
Bergeron RJ, Weimar WR, Luchetta G, Streiff RR, Wiegand SJ, Perrin J, Schreier KM, Porter C, Yao GW, Dimova H (1995) Metabolism and pharmacokinetics of N1, N11-diethylnorspermine. Drug Metab Dispos 23:1117–1125
Bergeron RJ, Feng Y, Weimar WR, McManis JS, Dimova H, Porter C, Raisler B, Phanstiel O (1997) A comparison of structure-activity relationships between spermidine and spermine analogue antineoplastics. J Med Chem 40:1475–1494
Bergeron RJ, Wiegand J, McManis JS, Weimar WR, Smith RE, Algee SE, Fannin TL, Slusher MA, Snyder PS (2001) Polyamine analogue antidiarrheals: a structure–activity study. J Med Chem 44:232–244
Bitonti AJ, Dumont JA, Bush TL, Edwards ML, Stemerick DM, McCann PP, Sjoerdsma A (1989) Bis(benzyl)polyamine analogs inhibit the growth of chloroquine-resistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with a-difluoromethylornithine cure murine malaria. Proc Natl Acad Sci USA 86:651–655
Bitonti AJ, Dumont JA, Bush TL, Stemerick DM, Edwards ML, McCann PP (1990) Bis(benzyl)polyamine analogs as novel substrates for polyamine oxidase. J Biol Chem 265:382–388
Bolkenius FN, Seiler N (1989) New substrates of polyamine oxidase. Dealkylation of N-alkyl-a, w-diamines. Biol Chem Hoppe Seyler 370:525–531
Byers TL, Bitonti AJ, McCann PP (1990) Bis(benzyl)polyamine analogues are substrates for a mammalian cell-transport system which is distinct from the polyamine-transport system. Biochem J 269:35–40
Casero RA Jr, Marton LJ (2007) Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 6:373–390
Devereux W, Wang Y, Stewart TM, Hacker A, Smith R, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Ward TD, Woster PM, Casero RA (2003) Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells. Cancer Chemother Pharmacol 52:383–390
Edwards ML, Stemerick DM, Bitonti AJ, Dumont JA, McCann PP, Bey P, Sjoerdsma A (1991) Antimalarial polyamine analogues. J Med Chem 34:569–574
FDA (2001) Guidance for industry, bioanalytical method validation. US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Available: http://www.fda.gov/cder/guidance/4252fnl.pdf
Heby O, Persson L, Rentala M (2007) Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of African sleeping sickness, Chagas’ disease, and leishmaniasis. Amino Acids 33:359–366
Hyvönen T, Keinänen TA, Khomutov AR, Khomutov RM, Eloranta TO (1992) Monitoring of the uptake and metabolism of aminooxy analogues of polyamines in cultured cells by high-performance liquid chromatography. J Chromatogr 574:17–21
Hyvönen MT, Keinänen TA, Cerrada-Gimenez M, Sinervirta R, Grigorenko N, Khomutov AR, Vepsäläinen J, Alhonen L, Jänne J (2007) Role of hypusinated eukaryotic translation initiation factor 5A in polyamine depletion-induced cytostasis. J Biol Chem 282:34700–34706
Häkkinen MR, Keinänen TA, Vepsäläinen J, Khomutov AR, Alhonen L, Jänne J, Auriola S (2007) Analysis of underivatized polyamines by reversed phase liquid chromatography with electrospray tandem mass spectrometry. J Pharm Biomed Anal 45:625–634
Häkkinen MR, Keinänen TA, Vepsäläinen J, Khomutov AR, Alhonen L, Jänne J, Auriola S (2008) Quantitative determination of underivatized polyamines by using isotope dilution RP-LC-ESI-MS/MS. J Pharm Biomed Anal 48:414–421
Häkkinen MR, Keinänen TA, Khomutov AR, Auriola S, Weisell J, Alhonen L, Jänne J, Vepsäläinen J (2009) Synthesis of novel deuterium labelled derivatives of N-alkylated polyamines. Tetrahedron 65:547–562
Hölttä E (1977) Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry 16:91–100
Järvinen A, Grigorenko N, Khomutov AR, Hyvönen MT, Uimari A, Vepsäläinen J, Sinervirta R, Keinänen TA, Vujcic S, Alhonen L, Porter CW, Jänne J (2005) Metabolic stability of alpha -methylated polyamine derivatives and their use as substitutes for the natural polyamines. J Biol Chem 280:6595–6601
Järvinen A, Keinänen TA, Grigorenko NA, Khomutov AR, Uimari A, Vepsäläinen J, Alhonen L, Jänne J (2006a) Guide molecule-driven stereospecific degradation of alpha-methylpolyamines by polyamine oxidase. J Biol Chem 281:4589–4595
Järvinen AJ, Cerrada-Gimenez M, Grigorenko NA, Khomutov AR, Vepsäläinen JJ, Sinervirta RM, Keinänen TA, Alhonen LI, Jänne JE (2006b) Alpha-methyl polyamines: efficient synthesis and tolerance studies in vivo and in vitro. First evidence for dormant stereospecificity of polyamine oxidase. J Med Chem 49:399–406
Kaiser A, Ulmer D, Goebel T, Holzgrabe U, Saeftel M, Hoerauf A (2006) Inhibition of hypusine biosynthesis in plasmodium: a possible, new strategy in prevention and therapy of malaria. Mini Rev Med Chem 6:1231–1241
Lawson KR, Marek S, Linehan JA, Woster PM, Casero RA Jr, Payne CM, Gerner EW (2002) Detoxification of the polyamine analogue N1-ethyl-N11-[(cycloheptyl)methy]-4, 8-diazaundecane (CHENSpm) by polyamine oxidase. Clin Cancer Res 8:1241–1247
Lee Y, Sayre LM (1998) Reaffirmation that metabolism of polyamines by bovine plasma amine oxidase occurs strictly at the primary amino termini. J Biol Chem 273:19490–19494
Lentini A, Mattioli P, Provenzano B, Abbruzzese A, Caraglia M, Beninati S (2007) Role of the FAD-dependent polyamine oxidase in the selective formation of N(1), N(8)-bis(gamma-glutamyl)spermidine protein cross-links. Biochem Soc Trans 35:396–400
Libby PR, Porter CW (1987) Separation of two isozymes of polyamine oxidase from murine L1210 leukemia cells. Biochem Biophys Res Commun 144:528–535
Muller IB, Das Gupta R, Luersen K, Wrenger C, Walter RD (2008) Assessing the polyamine metabolism of Plasmodium falciparum as chemotherapeutic target. Mol Biochem Parasitol 160:1–7
Pegg AE (1988) Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy. Cancer Res 48:759–774
Pegg AE, Feith DJ (2007) Polyamines and neoplastic growth. Biochem Soc Trans 35:295–299
Pegg AE, Poulin R, Coward JK (1995) Use of aminopropyltransferase inhibitors and of non-metabolizable analogs to study polyamine regulation and function. Int J Biochem Cell Biol 27:425–442
Pledgie A, Huang Y, Hacker A, Zhang Z, Woster PM, Davidson NE, Casero RA Jr (2005) Spermine oxidase SMO(PAOh1), Not N 1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J Biol Chem 280:39843–39851
Reguera RM, Tekwani BL, Balana-Fouce R (2005) Polyamine transport in parasites: a potential target for new antiparasitic drug development. Comp Biochem Physiol C Toxicol Pharmacol 140:151–164
Royo M, Fitzpatrick PF (2005) Mechanistic studies of mouse polyamine oxidase with N1, N12-bisethylspermine as a substrate. Biochemistry 44:7079–7084
Seiler N (1995) Polyamine oxidase, properties and functions. Prog Brain Res 106:333–344
Seiler N (2003) Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 2. Structural analogues and derivatives. Curr Drug Targets 4:565–585
Seiler N (2004) Catabolism of polyamines. Amino Acids 26:217–233
Smith RG, Daves DG Jr (1978) New mass spectrometric rearrangements involving silicon. A study of trimethylsilylated di- and polyamines and their isotopically labeled analogues. J Org Chem 43:2178–2183
Suzuki O, Matsumoto T, Katsumata Y (1984) Determination of polyamine oxidase activities in human tissues. Experientia 40:838–839
Tabor CW, Tabor H (1984) Polyamines. Ann Rev Biochem 53:749–790
Tsukada T, Furusako S, Maekawa S, Hibasami H, Nakashima K (1988) Purification by affinity chromatography and characterization of porcine liver cytoplasmic polyamine oxidase. Int J Biochem 20:695–702
Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW (2002) Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J 367:665–675
Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW (2003) Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J 370:19–28
Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA Jr (2001) Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res 61:5370–5373
Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, Casero RA Jr (2003) Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun 304:605–611
Wang Y, Hacker A, Murray-Stewart T, Frydman B, Valasinas A, Fraser AV, Woster PM, Casero RA Jr (2005a) Properties of recombinant human N1-acetylpolyamine oxidase (hPAO): potential role in determining drug sensitivity. Cancer Chemother Pharmacol 56:83–90
Wang Y, Hacker A, Murray-Stewart T, Fleischer JG, Woster PM, Casero RA Jr (2005b) Induction of human spermine oxidase SMO(PAOh1) is regulated at the levels of new mRNA synthesis, mRNA stabilization and newly synthesized protein. Biochem J 386:543–547
Williams K (1997) Interactions of polyamines with ion channels. Biochem J 325:289–297
Wu T, Yankovskaya V, McIntire WS (2003) Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N 1-acetylated polyamine oxidase. J Biol Chem 278:20514–20525
Acknowledgements
We thank Ms. Helena Vepsäläinen and Ms. Maritta Salminkoski, Department of Biosciences, Laboratory of Chemistry, University of Kuopio, for their help with LC-MS/MS sample preparation and in the synthesis work. Ms. Anne Karppinen and Ms. Tuula Reponen at A. I. Virtanen Institute, University of Kuopio, are acknowledged for their help with enzyme and cell experiments. This work was supported by Academy of Finland (projects 1241851 and 1287022), NIH (USA) CA984544, and the Russian Foundation for Basic Research (project 08-04-917775).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Häkkinen, M.R., Hyvönen, M.T., Auriola, S. et al. Metabolism of N-alkylated spermine analogues by polyamine and spermine oxidases. Amino Acids 38, 369–381 (2010). https://doi.org/10.1007/s00726-009-0429-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-009-0429-2