Abstract
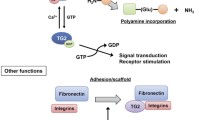
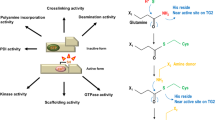
Transglutaminase 2 (TG2) is an inducible transamidating acyltransferase that catalyzes Ca2+-dependent protein modifications. It acts as a G protein in transmembrane signaling and as a cell surface adhesion mediator, this distinguishes it from other members of the transglutaminase family. The sequence motifs and domains revealed in the TG2 structure, can each be assigned distinct cellular functions, including the regulation of cytoskeleton, cell adhesion, and cell death. Though many biological functions of the enzyme have already been described or proposed previously, studies of TG2 null mice by our laboratory during the past years revealed several novel in vivo roles of the protein. In this review we will discuss these novel roles in their biological context.
Similar content being viewed by others
References
Akakura S, Singh S, Spataro M (2004) The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res 292:403–416
Akerman P, Cote P, Yang SQ et al (1992) Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol 263:G579–G585
Akimov SS, Krylov D, Fleischman LF et al (2000) Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol 148:825–838
André C, Couton D, Gaston J et al (1999) β2-Adrenergic receptor-selective agonist clenbuterol prevents Fas-induced liver apoptosis and death in mice. Am J Physiol 276:G647–G654
Balajthy Z, Csomós K, Vámosi G et al (2006) Tissue-transglutaminase contributes to neutrophil granulocyte differentiation and functions. Blood 108:2045–2054
Bernassola F, Federici M, Corazzari M et al (2002) Role of transglutaminase 2 in glucose tolerance: knockout mice studies and a putative mutation in a MODY patient. FASEB J 16:1371–1378
Bratosin D, Estaquier J, Petit F et al (2001) Programmed cell death in mature erythrocytes: a model for investigating death effector pathways operating in the absence of mitochondria. Cell Death Differ 8:1143–1156
Broten J, Michalopoulos G, Petersen B et al (1999) Adrenergic stimulation of hepatocyte growth factor expression. Biochem Biophys Res Commun 262:76–79
DeLaurenzi V, Melino G (2001) Gene disruption of tissue transglutaminase. Mol Cell Biol 21:148–155
Desbarats J, Newell MK (2000) Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med 6:920–923
Ellison RTIII, Giehl TJ (1991) Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest 88:1080–1091
Fadok VA, Voelker DR, Campbell PA et al (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148:2207–2216
Fadok VA, Bratton DL, Konowal A et al (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101:890–898
Falasca L, Iadevaia V, Ciccosanti F et al (2005) Transglutaminase type II is a key element in the regulation of the anti-inflammatory response elicited by apoptotic cell engulfment. J Immunol 174:7330–7740
Falasca L, Farrace MG, Rinaldi A et al (2008) Transglutaminase type II is involved in the pathogenesis of endotoxic shock. J Immunol 180:2616–2624
Fausto N, Campbell JS, Riehle KJ (2006) Liver regeneration. Hepatology 43:S45–S53
Fenchel G, Storf R, Michel J et al (1988) Relationship between the high-energy phosphate content and various left ventricular functional parameters of the normal and hypertrophied heart after global ischemia and reperfusion. Thorac Cardiovasc Surg 36:75–79
Feng JF, Rhee SG, Im MJ (1996) Evidence that phospholipase delta1 is the effector in the Gh (Transglutaminase II)-mediated signaling. J Biol Chem 271:16451–16454
Fésüs L, Piacentini M (2002) Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci 27:534–539
Fesus L, Tarcsa E (1989) Formation of N epsilon-(gamma-glutamyl)-lysine isodipeptide in Chinese-hamster ovary cells. Biochem J 263:843–848
Fesus L, Tarcsa E, Kedei N et al (1991) Degradation of cells dying by apoptosis leads to accumulation of epsilon(gamma-glutamyl)lysine isodipeptide in culture fluid and blood. FEBS Lett 284:109–112
Folk JE, Chung SI (1985) Transglutaminases. Methods Enzymol 113:358–375
Grenard P, Bates MK, Aeschlimann D (2001) Evolution of transglutaminase genes: identification of a transglutaminase gene cluster on human chromosome 15q15. Structure of the gene encoding transglutaminase X and a novel gene family member, transglutaminase Z. J Biol Chem 276:33066–33078
Hampton MB, Kettle AJ, Winterbourn CC (1998) Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007–3017
Hang J, Zemskov EA, Lorand L et al (2005) Identification of a novel recognition sequence for fibronectin within the NH2-terminal beta-sandwich domain of tissue transglutaminase. J Biol Chem 280:23675–23683
Hasegawa G, Suwa M, Ichikawa Y et al (2003) A novel function of tissue-type transglutaminase: protein disulphide isomerase. Biochem J 373:793–803
Herman JF, Mangala LS, Mehta K (2006) Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene 25:3049–3058
Iwai M, Cui TX, Kitamura H et al (2001) Increased secretion of tumour necrosis factor and interleukin 6 from isolated, perfused liver of rats after partial hepatectomy. Cytokine 13:60–64
Janiak A, Zemskov EA, Belkin AM (2006) Cell surface transglutaminase promotes RhoA activation via integrin clustering and suppression of the Src-p190RhoGAP signaling pathway. Mol Biol Cell 17:1606–1619
Jeremias I, Kupatt C, Martin-Villaba A et al (2000) Involvement of CD95/Apo/Fas in cell death after myocardial ischemia. Circulation 102:915–920
Kim HI, Rikihisa Y (2002) Roles of p38 mitogen-activated protein kinase, NF-kappaB, and protein kinase C in proinflammatory cytokine mRNA expression by human peripheral blood leukocytes, monocytes, and neutrophils in response to Anaplasma phagocytophila. Infect Immun 70:4132–4141
Kojima S, Nara K, Rifkin DB (1993) Requirement for transglutaminase in the activation of latent transforming growth factor-beta in bovine endothelial cells. J Cell Biol 121:439–448
Kosai K, Matsumoto K, Nagata S et al (1998) Abrogation of Fas-induced fulminant hepatic failure in mice by hepatocytes growth factor. Biochem Biophys Res Commun 244:683–690
Lanotte M, Martin-Thouvenin V, Najman S et al (1991) NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 77:1080–1086
Lopez-Boado YS, Espinola M, Bahr S et al (2004) Neutrophil serine proteinases cleave bacterial flagellin, abrogating its host response-inducing activity. J Immunol 72:509–515
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4:140–156
Maechler P, Wollheim CB (2001) Mitochondrial function in normal and diabetic beta-cells. Nature 414:807–812
Mastroberardino PG, Farrace MG, Viti I et al (2006) “Tissue” transglutaminase contributes to the formation of disulphide bridges in proteins of mitochondrial respiratory complexes. Biochim Biophys Acta 157:1357–1365
Michalopoulos GK, DeFrances MC (1997) Liver regeneration. Science 276:60–66
Mishra S, Melino G, Murphy LJ (2007) Transglutaminase 2 kinase activity facilitates protein kinase A-induced phosphorylation of retinoblastoma protein. J Biol Chem 282:18108–18115
Monsonego A, Friedmann I, Shani Y et al (1998) GTP-dependent conformational changes associated with the functional switch between Galpha and cross-linking activities in brain-derived tissue transglutaminase. J Mol Biol 282:713–720
Nakaoka H, Perez DM, Baek KJ et al (1994) Gh: a GTP binding protein with transglutaminase activity and receptor signaling function. Science 254:1593–1596
Nanda N, Iismaa SE, Owens WA et al (2001) Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem 276:20673–20678
Nardacci R, Lo Iacono O, Ciccosanti F et al (2003) Transglutaminase type II plays a protective role in hepatic injury. Am J Pathol 162:1293–1303
Nishiura H, Shibuya Y, Yamamoto T (1998) S19 ribosomal protein cross-linked dimer causes monocyte-predominant infiltration by means of molecular mimicry to complement C5a. Lab Invest 78:1615–1623
Piacentini M, Davies PJA, Fésüs L (1994) Tissue transglutaminase in cells undergoing apoptosis. In: Tomei LD, Cope FO (eds) Apoptosis. The molecular basis of cell death. Current communications in cell and molecular biology, vol. 3. Cold Spring Harbor Laboratory Press, New York
Piacentini M, Farrace MG, Piredda L et al (2002) Transglutaminase overexpression sensitizes neuronal cell lines to apoptosis by increasing mitochondrial membrane potential and cellular oxidative stress. J Neurochem 81:1061–1072
Piredda L, Amendola A, Colizzi V et al (1997) Lack of ‘tissue’ transglutaminase protein cross-linking leads to leakage of macromolecules from dying cells: relationship to development of autoimmunity in MRLlpr/lpr mice. Cell Death Differ 4:463–472
Ramarao CS, Denker JM, Perez DM et al (1992) Genomic organization and expression of the human alpha 1B-adrenergic receptor. J Biol Chem 267:21936–21945
Rodolfo C, Mormone E, Matarrese P et al (2004) Tissue transglutaminase is a multifunctional BH3-only protein. J Biol Chem 279:54783–54792
Rose DM, Fadok VA, Riches DW et al (1995) Autocrine/paracrine involvement of platelet-activating factor and transforming growth factor-beta in the induction of phosphatidylserine recognition by murine macrophages. J Immunol 155:5819–5825
Sarang Z, Molnár P, Németh T et al (2005) Tissue transglutaminase (TG2) acting as G protein protects hepatocytes against Fas-mediated cell death in mice. Hepatology 42:578–587
Sarang Z, Mádi A, Koy C et al (2007) Tissue transglutaminase (TG2) facilitates phosphatidylserine exposure and calpain activity in calcium-induced death of erythrocytes. Cell Death Differ 14:1842–1844
Stuart KA, Riordan SM, Lidder S et al (2000) Hepatocyte growth factor/scatter factor-induced intracellular signalling. Int J Exp Pathol 81:17–30
Szegezdi E, Szondy Z, Nagy L et al (2000) Apoptosis-linked in vivo regulation of the tissue transglutaminase gene promoter. Cell Death Differ 7:1225–1233
Szondy Z, Molnar P, Nemes Z et al (1997) Differential expression of tissue transglutaminase during in vivo apoptosis of thymocytes induced via distinct signalling pathways. FEBS Lett 104:307–313
Szondy Z, Sarang Z, Molnar P et al (2003) Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci USA 100:7812–7817
Szondy Z, Mastroberardino PG, Váradi J et al (2006) Tissue transglutaminase (TG2) protects cardiomyocytes against ischemia/reperfusion injury by regulating ATP synthesis. Cell Death Differ 13:1827–1829
Thomázy V, Fésüs L (1989) Differential expression of tissue transglutaminase in human cells. An immunohistochemical study. Cell Tissue Res 255:215–224
Upchurch HF, Conway E, Patterson MK et al (1991) Localization of cellular transglutaminase on the extracellular matrix after wounding: characteristics of the matrix bound enzyme. Cell Physiol 149:375–382
Webber EM, Bruix J, Pierce RH et al (1998) Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology 28:1226–1234
Wu J, Liu S-L, Zhu J-L (2000) Roles of tissue transglutaminase in ethanol-induced inhibition of hepatocyte proliferation and α1-adrenergic signal transduction. J Biol Chem 275:22213–22219
Yamaguchi S, Yamaoka M, Okuyama M et al (1999) Elevated circulating levels and cardiac secretion of soluble Fas ligand in patients with congestive heart failure. Am J Cardiol 83:1500–1503
Yin X-M, Wang Y, Gross A et al (1999) Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886–891
Acknowledgments
These studies were supported by Hungarian grants from the National Research Fund (OTKA T049445 and NI67877), Ministry of Welfare T (115/2006) and the EU HRTN-CT-2006-036032.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarang, Z., Tóth, B., Balajthy, Z. et al. Some lessons from the tissue transglutaminase knockout mouse. Amino Acids 36, 625–631 (2009). https://doi.org/10.1007/s00726-008-0130-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0130-x