Abstract
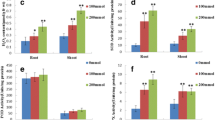
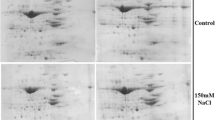
To evaluate the response of soybean to salt stress, the related changes in protein expression were investigated using the proteomic approach. Soybean plants were exposed to 0, 50, 100, and 200 mM NaCl. Especially at 200 mM, the length and fresh weight of the hypocotyl and root reduced under salt stress, while the proline content increased. Proteins from the hypocotyl and root treated with 100 mM NaCl were extracted and separated by two-dimensional polyacrylamide gel electrophoresis; 321 protein spots were detected. In response to salt stress, seven proteins were reproducibly found to be up- or down-regulated by two to sevenfold: late embryogenesis-abundant protein, β-conglycinin, elicitor peptide three precursor, and basic/helix-loop-helix protein were up-regulated, while protease inhibitor, lectin, and stem 31-kDa glycoprotein precursor were down-regulated. These results indicate that salinity can change the expression level of some special proteins in the hypocotyl and root of soybean that may in turn play a role in the adaptation to saline conditions.



Similar content being viewed by others
Abbreviations
- 2D-PAGE:
-
Two-dimensional polyacrylamide gel electrophoresis
References
Abbasi FM, Komatsu S (2004) A proteomic approach to analyze salt-responsive proteins in rice leaf sheath. Proteomics 4:2072–2081
Amini F, Ehsanpour A A, Hoang QT Shin JS (2007) Protein pattern changes in tomato under in vitro salt stress. Russ J Plant Physiol 54:464–471
Amitai-Zeigersona H, Scolnikb PA, Bar-Zvi D (1995) Tomato Asrl mRNA and protein are transiently expressed following salt stress, osmotic stress and treatment with abscisic acid. Plant Sci 110:205–213
Baker J, Steele C, Dure L (1988) Sequence and characterization of 6 Lea proteins and their genes from cotton. Plant Mol Biol 11:277–291
Banzai T, Hershkovits G, Katcoff DJ, Hanagata N, Dubinsky Z, Karube I (2002) Identification and characterization of mRNA transcrips differentially expressed in response to high salinity by means of differential display in the mangrove, Bruguiera gymnorrhiza. Plant Sci 162:499–505
Bates L, Waldren RP, Tear IP (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Benevides MP, Marconi LP, Gallego SM, Comba ME, Tomaro ML (2000) Relationship between antioxidant defense system and salt tolerance in Solanum tuberosum. Aus J Plant Physiol 27:273–278
Castro AJ, Carapito C, Zorn N, Magne C, Leize E, Dorsselaer AV (2005) Proteomic analysis of grapevine (Vitis vinifera L.) tissues subjected to herbicide stress. J Exp Bot 56:2783–2795
Chandler PM, Robertson M (1994) Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 45:113–141
Cleveland DW, Fischer SG, Krischer MW, Laemmli UK (1977) Peptide mapping by limited proteolysis in sodium dodecyl sulphate and analysis by gel electrophoresis. J Biol Chem 252:1102–1106
Ehsanpour AA, Fatahian N (2003) Effect of salt and proline on Medicago sativa callus. Plant Cell Tissue Organ Cult 73:53–56
Habib H, Fazili KM (2007) Plant protease inhibitors: a defense strategy in plants. Biotech Mol Biol Rev 2:68–85
Hajduch M, Rakwal R, Agrawal GK, Yonekura M, Pretova A (2001) High resolution two-dimensional electrophoresis separation of proteins from metal-stressed rice (Oryza sativa L.) leaves: drastic reductions/fragmentation of ribulose-1, 5-bisphosphate carboxylase/oxygenase and induction of stress-related proteins. Electrophoresis 22:2824–2831
Honee G, Buitink J, Jabs T, De Kloe J, Sijbolts F, Apotheker M, Weide R, Sijen T, Stuiver M, De Wit PJGM (1998) Induction of defense-related responses in Cf9 tomato cells by the AVR9 elicitor peptide of Cladosporium fulvum is developmentally regulated. Plant Physiol 117:809–820
Jelloulia N, Jouiraa HB, Skourib H, Ghorbela A, Gourgourib A, Mlikia A (2007) Proteomic analysis of Tunisian grapevine cultivar Razegui under salt stress. J Plant Physiol (on line)
Kao WY, Tsai TT, Tsai HC, Shih CN (2006) Response of three Glycine species to salt stress. Environ Exp Bot 56:120–125
Katerji N, Van Hoornb JW, Hamdyc A, Mastrorillid M (2000) Salt tolerance classification of crops according to soil salinity and to water stress day index. Agric Water Manag 43:99–109
Kong-Ngern K, Daduang S, Wongkham CH, Bunnag S, Kosittrakun M, Theerakulpisut P (2005) Protein profiles in response to salt stress in leaf sheaths of rice seedlings. Sci Asia 31:403–408
Krishman HB (2002) Evidence for accumulation of the β-subunit of β-conglycinin in soybean [Glycine max (L) Merr.] embryonic axes. Plant Cell Rep 20:869–875
Li D, Inoue H, Takahashi M, Kojima T, Shiraiwa M, Takahara H (2007) Molecular characterization of a novel salt inducible gene for an OSBP (oxysterol-binding protein)-homologue from soybean. Gene (on line)
Lu Z, Liu D, Liu S (2007) Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep (on line)
Luo Q, Yu B, Liu Y (2005) Differential sensitivity to chloride and sodium ions in seedlings of Glycine max and G. soja under NaCl stress. J Plant Physiol 162:1003–1012
Martienz CA, Maestri M, Lani EG (1996) In vitro salt tolerance and proline accumulation in Andean potato (Solanum spp.) differing in frost resistance. Plant Sci 116:177–184
Mittova V, Guy M, Tal M, Volokita M (2004) Salinity up-regulates the antioxidative system in root mithochondria and peroxisomes of wild salt-tolerant tomato species Lycopersicon pennellii. J Exp Bot 55:1105–1113
NDong C, Danyluk J, Wilson KE, Pocock T, Huner NP, Sarhan F (2002) Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins: molecular characterization and functional analyses. Plant Physiol 129:1368–1381
O’Farrell HP (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250:4007–4021
Onishi M, Tachi H, Kojima T, Shiraiwa M, Takahara H (2006) Molecular cloning and characterization of a novel salt inducible gene encoding an acidic isoform of PR-5 protein in soybean (Glycine max [L.] Merr). Plant Physiol Biochem 44:573–580
Parker R, Flowers TJ, Moore AL, Harpham NVJ (2006) An accurate and reproducible method for proteome profiling of the effects of salt stress in the rice leaf lamina. J Exp Bot 57:1109–1118
Peumans Willy J, Van Damme EJM (1995) Lectins as plant defense proteins. Plant Physiol 109:347–352
Rakwal R, Agrawal GK, Yonekura M (1999) Separation of proteins from stressed rice (Oryza sativa L.) leaf tissues by two-dimensional polyacrylamide gel electrophoresis: induction of pathogenesis-related and cellular protect proteins by jasmonic acid, UV irradiation and copper chloride. Electrophoresis 20:3472–3478
Sharifia M, Ghorbanlib M, Ebrahimzadeh H (2007) Improved growth of salinity-stressed soybean after inoculation with salt pre-treated mycorrhizal fungi. J Plant Physiol 164:1144–1151
Sharp RE, Hsiao TC, Silk WK (1990) Growth of maize primary root at low water potentials. Plant Physiol 93:1337–1346
Sha Valli Khan PS, Hoffmann L, Renaut J, Hausman JF (2007) Current initiatives in proteomics for the analysis of plant salt tolerance. Curr Sci 93:807–817
Soulages JL, Kim K, Walters C, Cushman JC (2002) Temperature-induced extended helix/random coil transitions in a group 1 late embryogenesis-abundant protein from soybean. Plant Physiol 128:822–832
Srivastava S, Fristensky B, Kav NNV (2004) Constitutive expression of a PR10 protein enhances the germination of Brassica napus under saline conditions. Plant Cell Physiol 45:1320–1324
Sule A, Vanrobaeys F, Hajos J, Beeumen JV, Devreese B (2004) Proteomic analysis of small heat shock protein isoforms in barley shoots. Phytochem 65:1853–1863
Toledo-Orits G, Huq E, Quail PH (2003) The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15:1749–1770
Umezawa T, Shimuzu K, Kato M, Ueda T (2000) Enhancement of salt tolerance in soybean with NaCl pretreatment. Physiol Plant 110:59–63
Van Hardeen PDR Kruger GHJ (2002) Separately and simultaneously induced dark chilling and drought stress effects on photosynthesis, proline accumulation and antioxidant metabolism in soybean. J Plant Physiol 159:1077–1086
Vincent D, Lapierre C, Pollet B, Cornic G, Negroni L, Zivy M (2005) Water deficits affect caffeate O-methyltransferase, lignification, and related enzymes in maize leaves: a proteomic investigation. Plant Physiol 3:949–960
Wang M, Gu D, Liu T, Wang Z, Guo Xiying, Hou W, Bai Y, Chen X, Wang G (2007) Overexpression of a putative maize calcineurin B-like protein in Arabidopsis confers salt tolerance. Plant Mol Biol (on line)
Xu D, Duan X, Wang B, Hong B, Ho THD, Wu R (1996) Expression of a late embryogenesis abundant protein gene, HVA7, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol 110:249–257
Acknowledgments
The authors are grateful to scholarship section of the ministry of Science, Research and technology of I. R. Iran and the higher education department of Isfahan University and also thank to the National Institute of Crop Science of Japan for their kindly supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghaei, K., Ehsanpour, A.A., Shah, A.H. et al. Proteome analysis of soybean hypocotyl and root under salt stress. Amino Acids 36, 91–98 (2009). https://doi.org/10.1007/s00726-008-0036-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0036-7