Summary.
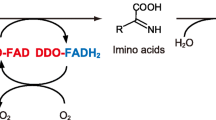
The role of Ser-308 of murine d-aspartate oxidase (mDASPO), particularly its side chain hydroxyl group, was investigated through the use of site-specific mutational analysis of Ser-308. Recombinant mDASPO carrying a substitution of Gly, Ala, or Tyr for Ser-308 was generated, and fused to either His (His-mDASPO), or glutathione S-transferase, His, and S (GHS-mDASPO) at its N-terminus. Wild-type His-mDASPO or GHS-mDASPO or their mutant derivatives were expressed in Escherichia coli and purified by affinity chromatography. All purified recombinant proteins had functional DASPO activity. The Gly-308 and Ala-308 mutants had significantly higher catalytic efficiency towards d-Asp and N-methyl-d-Asp, and a higher affinity for flavin adenine dinucleotide (FAD) compared to the wild-type enzyme. The Tyr-308 mutant had lower catalytic efficiency and binding capacity. These results suggest that the side chain hydroxyl group of a critical residue of mDASPO, Ser-308, down-regulates enzymatic activity, substrate binding, and FAD binding. This study provides information on the active site of DASPO that will considerably enhance our understanding of the biological significance of this enzyme.
Similar content being viewed by others
Abbreviations
- DAAO:
-
d-amino acid oxidase
- DASPO:
-
d-aspartate oxidase
- FAD:
-
flavin adenine dinucleotide
- GHS:
-
glutathione S-transferase, His, and S
- GHS-mDASPO:
-
N-terminally glutathione S-transferase-, His-, and S-tagged mouse d-aspartate oxidase
- GST:
-
glutathione S-transferase
- His-mDASPO:
-
N-terminally His-tagged mouse d-aspartate oxidase
- K d, FAD :
-
dissociation constant value for binding to flavin adenine dinucleotide
- mDASPO:
-
mouse d-aspartate oxidase
- NMDA:
-
N-methyl-d-aspartate
References
H Abe N Yoshikawa MG Sarower S Okada (2005) ArticleTitlePhysiological function and metabolism of free D-alanine in aquatic animals Biol Pharm Bull 28 1571–1577 Occurrence Handle16141518 Occurrence Handle10.1248/bpb.28.1571 Occurrence Handle1:CAS:528:DC%2BD2MXhtFWjurzK
A Boselli L Piubelli G Molla S Sacchi MS Pilone S Ghisla L Pollegioni (2004) ArticleTitleOn the mechanism of Rhodotorula gracilis D-amino acid oxidase: role of the active site serine 335 Biochim Biophys Acta 1702 19–32 Occurrence Handle15450847 Occurrence Handle1:CAS:528:DC%2BD2cXnvVOiurw%3D
MM Bradford (1976) ArticleTitleA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding Anal Biochem 72 248–254 Occurrence Handle942051 Occurrence Handle10.1016/0003-2697(76)90527-3 Occurrence Handle1:CAS:528:DyaE28XksVehtrY%3D
L Caldinelli G Molla MS Pilone L Pollegioni (2006) ArticleTitleTryptophan 243 affects interprotein contacts, cofactor binding and stability in D-amino acid oxidase from Rhodotorula gracilis FEBS J 273 504–512 Occurrence Handle16420474 Occurrence Handle10.1111/j.1742-4658.2005.05083.x Occurrence Handle1:CAS:528:DC%2BD28Xis1Giu78%3D
P Casalin L Pollegioni B Curti MS Pilone (1991) ArticleTitleA study on apoenzyme from Rhodotorula gracilis D-amino acid oxidase Eur J Biochem 197 513–517 Occurrence Handle1673927 Occurrence Handle10.1111/j.1432-1033.1991.tb15939.x Occurrence Handle1:CAS:528:DyaK3MXktVeqsb8%3D
A D’Aniello (2007) ArticleTitleD-Aspartic acid: an endogenous amino acid with an important neuroendocrine role Brain Res Rev 53 215–234 Occurrence Handle17118457 Occurrence Handle10.1016/j.brainresrev.2006.08.005 Occurrence Handle1:CAS:528:DC%2BD2sXhtlClurk%3D
F Errico MT Pirro A Affuso P Spinelli M De Felice A D’Aniello R Di Lauro (2006) ArticleTitleA physiological mechanism to regulate D-aspartic acid and NMDA levels in mammals revealed by D-aspartate oxidase deficient mice Gene 374 50–57 Occurrence Handle16516413 Occurrence Handle10.1016/j.gene.2006.01.010 Occurrence Handle1:CAS:528:DC%2BD28XkvVWrsr8%3D
N Fujii (2005) ArticleTitleD-Amino acid in elderly tissues Biol Pharm Bull 28 1585–1589 Occurrence Handle16141520 Occurrence Handle10.1248/bpb.28.1585 Occurrence Handle1:CAS:528:DC%2BD2MXhtFWjurzE
K Hamase R Konno A Morikawa K Zaitsu (2005) ArticleTitleSensitive determination of D-amino acids in mammals and the effect of D-amino-acid oxidase activity on their amounts Biol Pharm Bull 28 1578–1584 Occurrence Handle16141519 Occurrence Handle10.1248/bpb.28.1578 Occurrence Handle1:CAS:528:DC%2BD2MXhtFWjurzL
A Hashimoto T Nishikawa R Konno A Niwa Y Yasumura T Oka K Takahashi (1993) ArticleTitleFree D-serine, D-aspartate and D-alanine in central nervous system and serum in mutant mice lacking D-amino acid oxidase Neurosci Lett 152 33–36 Occurrence Handle8100053 Occurrence Handle10.1016/0304-3940(93)90476-2 Occurrence Handle1:CAS:528:DyaK3sXkvFWqtb8%3D
H Homma (2007) ArticleTitleBiochemistry of D-aspartate in mammalian cells Amino Acids 32 3–11 Occurrence Handle16755369 Occurrence Handle10.1007/s00726-006-0354-6 Occurrence Handle1:CAS:528:DC%2BD28Xht1Ogs7vJ
AS Huang A Beigneux ZM Weil PM Kim ME Molliver S Blackshaw RJ Nelson SG Young SH Snyder (2006) ArticleTitleD-Aspartate regulates melanocortin formation and function: behavioral alterations in D-aspartate oxidase-deficient mice J Neurosci 26 2814–2819 Occurrence Handle16525061 Occurrence Handle10.1523/JNEUROSCI.5060-05.2006 Occurrence Handle1:CAS:528:DC%2BD28XislKrurw%3D
I Iwazaki S Yamashita K Arimoto M Takahashi Y Kera R Yamada (2000) ArticleTitleApoenzyme from Cryptococcus humicolus UJ1 D-aspartate oxidase J Mol Catal B 10 183–189 Occurrence Handle10.1016/S1381-1177(00)00127-2 Occurrence Handle1:CAS:528:DC%2BD3cXltVGqs78%3D
M Katane T Furuchi M Sekine H Homma (2007a) ArticleTitleMolecular cloning of a cDNA encoding mouse D-aspartate oxidase and functional characterization of its recombinant proteins by site-directed mutagenesis Amino Acids 32 69–78 Occurrence Handle10.1007/s00726-006-0350-x Occurrence Handle1:CAS:528:DC%2BD28Xht1Ogs7rF
M Katane Y Seida M Sekine T Furuchi H Homma (2007b) ArticleTitle Caenorhabditis elegans has two genes encoding functional D-aspartate oxidases FEBS J 274 137–149 Occurrence Handle10.1111/j.1742-4658.2006.05571.x Occurrence Handle1:CAS:528:DC%2BD2sXmsFyrsQ%3D%3D
T Kawazoe H Tsuge MS Pilone K Fukui (2006) ArticleTitleCrystal structure of human D-amino acid oxidase: context-dependent variability of the backbone conformation of the VAAGL hydrophobic stretch located at the si-face of the flavin ring Protein Sci 15 2708–2717 Occurrence Handle17088322 Occurrence Handle10.1110/ps.062421606 Occurrence Handle1:CAS:528:DC%2BD28XhtlSmtb%2FF
R Konno Y Yasumura (1983) ArticleTitleMouse mutant deficient in D-amino acid oxidase activity Genetics 103 277–285 Occurrence Handle6131852 Occurrence Handle1:CAS:528:DyaL3sXos1ylsw%3D%3D
V Massey H Ganther (1965) ArticleTitleOn the interpretation of the absorption spectra of flavoproteins with special reference to D-amino acid oxidase Biochemistry 4 1161–1173 Occurrence Handle4378783 Occurrence Handle10.1021/bi00882a027 Occurrence Handle1:CAS:528:DyaF2MXktFyisL8%3D
A Mattevi MA Vanoni F Todone M Rizzi A Teplyakov A Coda M Bolognesi B Curti (1996) ArticleTitleCrystal structure of D-amino acid oxidase: a case of active site mirror-image convergent evolution with flavocytochrome b 2 Proc Natl Acad Sci USA 93 7496–7501 Occurrence Handle8755502 Occurrence Handle10.1073/pnas.93.15.7496 Occurrence Handle1:CAS:528:DyaK28XksFSku7k%3D
R Miura C Setoyama Y Nishina K Shiga H Mizutani I Miyahara K Hirotsu (1997) ArticleTitleStructural and mechanistic studies on D-amino acid oxidase-substrate complex: implications of the crystal structure of enzyme-substrate analog complex J Biochem (Tokyo) 122 825–833 Occurrence Handle1:CAS:528:DyaK2sXnt1ygsbk%3D
H Mizutani I Miyahara K Hirotsu Y Nishina K Shiga C Setoyama R Miura (1996) ArticleTitleThree-dimensional structure of porcine kidney D-amino acid oxidase at 3.0 Å resolution J Biochem (Tokyo) 120 14–17 Occurrence Handle1:CAS:528:DyaK28XltFGltr8%3D
H Mizutani I Miyahara K Hirotsu Y Nishina K Shiga C Setoyama R Miura (2000) ArticleTitleThree-dimensional structure of the purple intermediate of porcine kidney D-amino acid oxidase. Optimization of the oxidative half-reaction through alignment of the product with reduced flavin J Biochem (Tokyo) 128 73–81 Occurrence Handle1:CAS:528:DC%2BD3cXmtVegsbw%3D
G Molla S Sacchi M Bernasconi MS Pilone K Fukui L Pollegioni (2006) ArticleTitleCharacterization of human D-amino acid oxidase FEBS Lett 580 2358–2364 Occurrence Handle16616139 Occurrence Handle10.1016/j.febslet.2006.03.045 Occurrence Handle1:CAS:528:DC%2BD28Xjs1aisrs%3D
A Negri V Massey CH Williams SuffixJr (1987) ArticleTitleD-Aspartate oxidase from beef kidney. Purification and properties J Biol Chem 262 10026–10034 Occurrence Handle3611051 Occurrence Handle1:CAS:528:DyaL2sXkvFamsrs%3D
A Negri V Massey CH Williams SuffixJr LM Schopfer (1988) ArticleTitleThe kinetic mechanism of beef kidney D-aspartate oxidase J Biol Chem 263 13557–13563 Occurrence Handle2901415 Occurrence Handle1:CAS:528:DyaL1cXlvVKhsLo%3D
MS Pilone (2000) ArticleTitleD-Amino acid oxidase: new findings Cell Mol Life Sci 57 1732–1747 Occurrence Handle11130179 Occurrence Handle10.1007/PL00000655 Occurrence Handle1:CAS:528:DC%2BD3MXhsFKgsA%3D%3D
S Sacchi S Lorenzi G Molla MS Pilone C Rossetti L Pollegioni (2002) ArticleTitleEngineering the substrate specificity of D-amino-acid oxidase J Biol Chem 277 27510–27516 Occurrence Handle12021281 Occurrence Handle10.1074/jbc.M203946200 Occurrence Handle1:CAS:528:DC%2BD38XlvV2hsrw%3D
MJ Schell (2004) ArticleTitleThe N-methyl D-aspartate receptor glycine site and D-serine metabolism: an evolutionary perspective Phil Trans R Soc London Ser B 359 943–964 Occurrence Handle10.1098/rstb.2003.1399 Occurrence Handle1:CAS:528:DC%2BD2cXmt1yms7k%3D
MJ Schell OB Cooper SH Snyder (1997) ArticleTitleD-Aspartate localizations imply neuronal and neuroendocrine roles Proc Natl Acad Sci USA 94 2013–2018 Occurrence Handle9050896 Occurrence Handle10.1073/pnas.94.5.2013 Occurrence Handle1:CAS:528:DyaK2sXhslertro%3D
MJ Schell ME Molliver SH Snyder (1995) ArticleTitleD-Serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release Proc Natl Acad Sci USA 92 3948–3952 Occurrence Handle7732010 Occurrence Handle10.1073/pnas.92.9.3948 Occurrence Handle1:CAS:528:DyaK2MXlsVSks7k%3D
MJ Scolari GB Acosta (2007) ArticleTitleD-Serine: a new word in the glutamatergic neuro-glial language Amino Acids 33 563–574 Occurrence Handle17245616 Occurrence Handle10.1007/s00726-006-0481-0 Occurrence Handle1:CAS:528:DC%2BD2sXhtlSlsrnF
C Setoyama Y Nishina H Mizutani I Miyahara K Hirotsu N Kamiya K Shiga R Miura (2006) ArticleTitleEngineering the substrate specificity of porcine kidney D-amino acid oxidase by mutagenesis of the “active-site lid” J Biochem (Tokyo) 139 873–879 Occurrence Handle1:CAS:528:DC%2BD28XmtlKqt74%3D
C Setoyama Y Nishina H Tamaoki H Mizutani I Miyahara K Hirotsu K Shiga R Miura (2002) ArticleTitleEffects of hydrogen bonds in association with flavin and substrate in flavoenzyme D-amino acid oxidase. The catalytic and structural roles of Gly313 and Thr317 J Biochem (Tokyo) 131 59–69 Occurrence Handle1:CAS:528:DC%2BD38Xitl2qs78%3D
G Tedeschi A Negri F Ceciliani S Ronchi A Vetere G D’Aniello A D’Aniello (1994) ArticleTitleProperties of the flavoenzyme D-aspartate oxidase from Octopus vulgaris Biochim Biophys Acta 1207 217–222 Occurrence Handle7915543 Occurrence Handle1:CAS:528:DyaK2MXltVyitg%3D%3D
S Umhau L Pollegioni G Molla K Diederichs W Welte MS Pilone S Ghisla (2000) ArticleTitleThe X-ray structure of D-amino acid oxidase at very high resolution identifies the chemical mechanism of flavin-dependent substrate dehydrogenation Proc Natl Acad Sci USA 97 12463–12468 Occurrence Handle11070076 Occurrence Handle10.1073/pnas.97.23.12463 Occurrence Handle1:CAS:528:DC%2BD3cXotFyhsr4%3D
A Yamamoto H Tanaka T Ishida K Horiike (2007) ArticleTitleFunctional and structural characterization of D-aspartate oxidase from porcine kidney: non-Michaelis kinetics due to substrate activation J Biochem (Tokyo) 141 363–376 Occurrence Handle1:CAS:528:DC%2BD2sXlsVagtbg%3D
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ address: Hiroshi Homma, Laboratory of Biomolecular Science, School of Pharmaceutical Sciences, Kitasato University, 5-9-1 Shirokane, Minato-ku, Tokyo 108-8641, Japan
Rights and permissions
About this article
Cite this article
Katane, M., Hanai, T., Furuchi, T. et al. Hyperactive mutants of mouse d-aspartate oxidase: mutagenesis of the active site residue serine 308. Amino Acids 35, 75–82 (2008). https://doi.org/10.1007/s00726-007-0627-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-007-0627-8