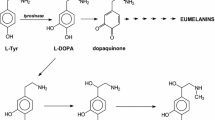
Summary.
The effects of dioxygen on tyrosine hydroxylase (TH) activity was studied, measuring the formation of DOPA from tyrosine, 3H2O from 3,5-3H-tyrosine, or by direct oxygraphic determination of oxygen consumption. A high enzyme activity was observed during the initial 1–2 min of the reactions, followed by a decline in activity, possibly related to a turnover dependent substoichiometrical oxidation of enzyme bound Fe(II) to the inactive Fe(III) state. During the initial reaction phase, apparent K m-values of 29–45 µM for dioxygen were determined for all human TH isoforms, i.e. 2–40 times higher than previously reported for TH isolated from animal tissues. After 8 min incubation, the K m (O2)-values had declined to an average of 20 ± 4 µM. Thus, TH activity may be severely limited by oxygen availability even at moderate hypoxic conditions, and the enzyme is rapidly and turnover dependent inactivated at the experimental conditions commonly employed to measure in vitro activities.
Similar content being viewed by others
Abbreviations
- BH4 :
-
(6R)-L-erythro-5,6,7,8-tetrahydrobiopterin
- 4aOH-BH4 :
-
4a-hydroxytetrahydrobiopterin
- q-BH2 :
-
quinonoid dihydrobiopterin
- DTT:
-
dithiothreitol
- hTH:
-
human tyrosine hydroxylase
- PAH:
-
phenylalanine hydroxylase
- TH:
-
tyrosine hydroxylase
- Enzymes: phenylalanine hydroxylase:
-
EC 1.14.16.1
- tyrosine hydroxylase:
-
EC 1.14.16.2
References
B Almas B Le Bourdelles T Flatmark J Mallet J Haavik (1992) ArticleTitleRegulation of recombinant human tyrosine hydroxylase isozymes by catecholamine binding and phosphorylation. Structure/activity studies and mechanistic implications Eur J Biochem 209 249–255 Occurrence Handle1356768 Occurrence Handle10.1111/j.1432-1033.1992.tb17283.x Occurrence Handle1:STN:280:DyaK3s%2FhtFWgsQ%3D%3D
KK Andersson C Vassort BA Brennan L Que SuffixJr J Haavik T Flatmark F Gros J Thibault (1992) ArticleTitlePurification and characterization of the blue-green rat phaeochromocytoma (PC12) tyrosine hydroxylase with a dopamine-Fe(III) complex. Reversal of the endogenous feedback inhibition by phosphorylation of serine-40 Biochem J 284 687–695 Occurrence Handle1352446 Occurrence Handle1:CAS:528:DyaK38XksVehtbs%3D
GE Bisgard (1995) ArticleTitleIncrease in carotid body sensitivity during sustained hypoxia Biol Signals 4 292–297 Occurrence Handle8704830 Occurrence Handle10.1159/000109455 Occurrence Handle1:CAS:528:DyaK28XovV2lsw%3D%3D
RM Brown W Kehr A Carlsson (1975) ArticleTitleFunctional and biochemical aspects of catecholamine metabolism in brain under hypoxia Brain Res 85 491–509 Occurrence Handle236073 Occurrence Handle10.1016/0006-8993(75)90822-7 Occurrence Handle1:CAS:528:DyaE2MXhtlKks7g%3D
MF Czyzyk-Krzeska BA Furnari EE Lawson DE Millhorn (1994) ArticleTitleHypoxia increases rate of transcription and stability of tyrosine hydroxylase mRNA in pheochromocytoma (PC12) cells J Biol Chem 269 760–764 Occurrence Handle7903970 Occurrence Handle1:CAS:528:DyaK2cXnsVyisA%3D%3D
JN Davis (1976) ArticleTitleBrain tyrosine hydroxylation: alteration of oxygen affinity in vivo by immobilization or electroshock in the rat J Neurochem 27 211–215 Occurrence Handle8590 Occurrence Handle10.1111/j.1471-4159.1976.tb01566.x Occurrence Handle1:CAS:528:DyaE28XlvV2kur8%3D
JN Davis A Carlsson (1973) ArticleTitleEffect of hypoxia on tyrosine and tryptophan hydroxylation in unanaesthetized rat brain J Neurochem 20 913–915 Occurrence Handle4703804 Occurrence Handle10.1111/j.1471-4159.1973.tb00055.x Occurrence Handle1:CAS:528:DyaE3sXktVentbY%3D
SH Feinsilver R Wong DM Raybin (1987) ArticleTitleAdaptations of neurotransmitter synthesis to chronic hypoxia in cell culture Biochim Biophys Acta 928 56–62 Occurrence Handle2881582 Occurrence Handle10.1016/0167-4889(87)90085-1 Occurrence Handle1:CAS:528:DyaL2sXitFWqt7w%3D
DB Fisher S Kaufman (1972) ArticleTitleThe inhibition of phenylalanine and tyrosine hydroxylases by high oxygen levels J Neurochem 19 1359–1365 Occurrence Handle4401997 Occurrence Handle10.1111/j.1471-4159.1972.tb01460.x Occurrence Handle1:CAS:528:DyaE38XksVCksLg%3D
PF Fitzpatrick (1991) ArticleTitleSteady-state kinetic mechanism of rat tyrosine hydroxylase Biochemistry 30 3658–3662 Occurrence Handle1673058 Occurrence Handle10.1021/bi00229a010 Occurrence Handle1:CAS:528:DyaK3MXhvVWitbo%3D
PF Fitzpatrick (2003) ArticleTitleMechanism of aromatic amino acid hydroxylation Biochemistry 42 14083–14091 Occurrence Handle14640675 Occurrence Handle10.1021/bi035656u Occurrence Handle1:CAS:528:DC%2BD3sXovVOiu74%3D
PF Fitzpatrick LJ Chlumsky SC Daubner KL O’Malley (1990) ArticleTitleExpression of rat tyrosine hydroxylase in insect tissue culture cells and purification and characterization of the cloned enzyme J Biol Chem 265 2042–2047 Occurrence Handle1967606 Occurrence Handle1:CAS:528:DyaK3cXhtFCltr4%3D
T Flatmark B Almas PM Knappskog SV Berge RM Svebak R Chehin A Muga A Martinez (1999) ArticleTitleTyrosine hydroxylase binds tetrahydrobiopterin cofactor with negative cooperativity, as shown by kinetic analyses and surface plasmon resonance detection Eur J Biochem 262 840–849 Occurrence Handle10411647 Occurrence Handle10.1046/j.1432-1327.1999.00445.x Occurrence Handle1:CAS:528:DyaK1MXkt1yru78%3D
A Fossbakk J Haavik (2005) ArticleTitleAn oxygraphic method for determining kinetic properties and catalytic mechanism of aromatic amino acid hydroxylases Anal Biochem 343 100–105 Occurrence Handle15963939 Occurrence Handle1:CAS:528:DC%2BD2MXmtFSnur8%3D Occurrence Handle10.1016/j.ab.2005.04.043
PA Frantom J Seravalli SW Ragsdale PF Fitzpatrick (2006) ArticleTitleReduction and oxidation of the active site iron in tyrosine hydroxylase: kinetics and specificity Biochemistry 45 4338 Occurrence Handle10.1021/bi0680072 Occurrence Handle1:CAS:528:DC%2BD28XitFOitLs%3D
E Gnaiger R Steinlechner-Maran G Mendez T Eberl R Margreiter (1995) ArticleTitleControl of mitochondrial and cellular respiration by oxygen J Bioenerg Biomembr 27 583–596 Occurrence Handle8746845 Occurrence Handle10.1007/BF02111656 Occurrence Handle1:CAS:528:DyaK28XhtF2gtw%3D%3D
KE Goodwill C Sabatier C Marks R Raag PF Fitzpatrick RC Stevens (1997) ArticleTitleCrystal structure of tyrosine hydroxylase at 2.3 A and its implications for inherited neurodegenerative diseases Nat Struct Biol 4 578–585 Occurrence Handle9228951 Occurrence Handle10.1038/nsb0797-578 Occurrence Handle1:CAS:528:DyaK2sXksVeks7Y%3D
E Gozal ZA Shah JM Pequignot J Pequignot LR Sachleben MF Czyzyk-Krzeska RC Li SZ Guo D Gozal (2005) ArticleTitleTyrosine hydroxylase expression and activity in the rat brain: differential regulation after long-term intermittent or sustained hypoxia J Appl Physiol 99 642–649 Occurrence Handle15817718 Occurrence Handle10.1152/japplphysiol.00880.2004 Occurrence Handle1:CAS:528:DC%2BD2MXpsVaqs7o%3D
B Grima A Lamouroux C Boni JF Julien F Javoy-Agid J Mallet (1987) ArticleTitleA single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics Nature 326 707–711 Occurrence Handle2882428 Occurrence Handle10.1038/326707a0 Occurrence Handle1:CAS:528:DyaL2sXkslWrur8%3D
J Haavik KK Andersson L Petersson T Flatmark (1988) ArticleTitleSoluble tyrosine hydroxylase (tyrosine 3-monooxygenase) from bovine adrenal medulla: large-scale purification and physicochemical properties Biochim Biophys Acta 953 142–156 Occurrence Handle2894860 Occurrence Handle1:CAS:528:DyaL1cXhvValtrY%3D
J Haavik T Flatmark (1980) ArticleTitleRapid and sensitive assay of tyrosine 3-monooxygenase activity by high-performance liquid chromatography using the native fluorescence of DOPA J Chromatogr 198 511–515 Occurrence Handle6108329 Occurrence Handle10.1016/S0021-9673(00)80522-1 Occurrence Handle1:CAS:528:DyaL3cXmtlems7k%3D
J Haavik T Flatmark (1983) ArticleTitleIsolation and characterization of quinonoid dihydropterins by high-performance liquid chromatography J Chromatogr 257 361–372 Occurrence Handle10.1016/S0021-9673(01)88192-9 Occurrence Handle1:CAS:528:DyaL3sXktlamsbs%3D
J Haavik T Flatmark (1987) ArticleTitleIsolation and characterization of tetrahydropterin oxidation products generated in the tyrosine 3-monooxygenase (tyrosine hydroxylase) reaction Eur J Biochem 168 21–26 Occurrence Handle2889594 Occurrence Handle10.1111/j.1432-1033.1987.tb13381.x Occurrence Handle1:CAS:528:DyaL1cXisF2q
J Haavik B Le Bourdelles A Martinez T Flatmark J Mallet (1991) ArticleTitleRecombinant human tyrosine hydroxylase isozymes. Reconstitution with iron and inhibitory effect of other metal ions Eur J Biochem 199 371–378 Occurrence Handle1676967 Occurrence Handle10.1111/j.1432-1033.1991.tb16133.x Occurrence Handle1:CAS:528:DyaK3MXltlSms74%3D
J Haavik A Martinez S Olafsdottir J Mallet T Flatmark (1992) ArticleTitleThe incorporation of divalent metal ions into recombinant human tyrosine hydroxylase apoenzymes studied by intrinsic fluorescence and 1H-NMR spectroscopy Eur J Biochem 210 23–31 Occurrence Handle1359966 Occurrence Handle10.1111/j.1432-1033.1992.tb17386.x Occurrence Handle1:CAS:528:DyaK38Xmt1yqsbk%3D
Y Hayashi S Miwa K Lee K Koshimura K Hamahata H Hasegawa M Fujiwara Y Watanabe (1990) ArticleTitleEnhancement of in vivo tyrosine hydroxylation in the rat adrenal gland under hypoxic conditions J Neurochem 54 1115–1121 Occurrence Handle1968954 Occurrence Handle10.1111/j.1471-4159.1990.tb01937.x Occurrence Handle1:CAS:528:DyaK3cXitlenurw%3D
M Hirsila P Koivunen V Gunzler KI Kivirikko J Myllyharju (2003) ArticleTitleCharacterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor J Biol Chem 278 30772–30780 Occurrence Handle12788921 Occurrence Handle10.1074/jbc.M304982200 Occurrence Handle1:CAS:528:DC%2BD3sXmtFeqsb4%3D
M Ikeda LA Fahien S Udenfriend (1966) ArticleTitleA kinetic study of bovine adrenal tyrosine hydroxylase J Biol Chem 241 4452–4456 Occurrence Handle5922968 Occurrence Handle1:CAS:528:DyaF28XkslCqtLY%3D
IR Katz (1980) ArticleTitleOxygen affinity of tyrosine and tryptophan hydroxylases in synaptosomes J Neurochem 35 760–763 Occurrence Handle6108999 Occurrence Handle10.1111/j.1471-4159.1980.tb03721.x Occurrence Handle1:CAS:528:DyaL3cXls1arurY%3D
M Knappskog HG Eiken A Martinez S Olafsdottir J Haavik T Flatmark J Apold (1993) ArticleTitleExpression of wild type and mutant forms of human phenylalanine hydroxylase in E. coli Adv Exp Med Biol 338 59–62 Occurrence Handle8304187 Occurrence Handle1:CAS:528:DyaK2MXkslagsQ%3D%3D
R Koch (1996) ArticleTitleTyrosine supplementation for phenylketonuria treatment Am J Clin Nutr 64 974–975 Occurrence Handle8942426 Occurrence Handle1:STN:280:DyaK2s%2Fpslyrtw%3D%3D
DM Kuhn CW Aretha TJ Geddes (1999) ArticleTitlePeroxynitrite inactivation of tyrosine hydroxylase: mediation by sulfhydryl oxidation, not tyrosine nitration J Neurosci 19 10289–10294 Occurrence Handle10575026 Occurrence Handle1:CAS:528:DyaK1MXnslyrt7k%3D
U Leuenberger K Gleeson K Wroblewski S Prophet R Zelis C Zwillich L Sinoway (1991) ArticleTitleNorepinephrine clearance is increased during acute hypoxemia in humans Am J Physiol 261 H1659–H1664 Occurrence Handle1951753 Occurrence Handle1:CAS:528:DyaK38XjvV2itw%3D%3D
W Meyer-Klaucke H Winkler V Schunemann AX Trautwein HF Nolting J Haavik (1996) ArticleTitleMossbauer, electron-paramagnetic-resonance and X-ray-absorption fine-structure studies of the iron environment in recombinant human tyrosine hydroxylase Eur J Biochem 241 432–439 Occurrence Handle8917440 Occurrence Handle10.1111/j.1432-1033.1996.00432.x Occurrence Handle1:CAS:528:DyaK28XmvFeru70%3D
Y Numata T Kato T Nagatsu T Sugimoto S Matsuura (1977) ArticleTitleEffects of stereochemical structures of tetrahydrobiopterin on tyrosine hydroxylase Biochim Biophys Acta 480 104–112 Occurrence Handle12820 Occurrence Handle1:CAS:528:DyaE2sXmslCjtA%3D%3D
AJ Ramsey PJ Hillas PF Fitzpatrick (1996) ArticleTitleCharacterization of the active site iron in tyrosine hydroxylase. Redox states of the iron J Biol Chem 271 24395–24400 Occurrence Handle8798695 Occurrence Handle10.1074/jbc.271.40.24395 Occurrence Handle1:CAS:528:DyaK28XmtFeitrs%3D
EL Rolett A Azzawi KJ Liu MN Yongbi HM Swartz JF Dunn (2000) ArticleTitleCritical oxygen tension in rat brain: a combined (31)P-NMR and EPR oximetry study Am J Physiol Regul Integr Comp Physiol 279 R9–R16 Occurrence Handle10896858 Occurrence Handle1:CAS:528:DC%2BD3cXltlyqsr0%3D
M Rostrup (1998) ArticleTitleCatecholamines, hypoxia and high altitude Acta Physiol Scand 162 389–399 Occurrence Handle9578385 Occurrence Handle10.1046/j.1365-201X.1998.00335.x Occurrence Handle1:CAS:528:DyaK1cXitlGqtb0%3D
K Sevre B Bendz E Hanko AR Nakstad A Hauge JI Kasin JD Lefrandt AJ Smit I Eide M Rostrup (2001) ArticleTitleReduced autonomic activity during stepwise exposure to high altitude Acta Physiol Scand 173 409–417 Occurrence Handle11903133 Occurrence Handle10.1046/j.1365-201X.2001.00925.x Occurrence Handle1:CAS:528:DC%2BD38XhslyrtA%3D%3D
O Siggaard-Andersen N Fogh-Andersen IH Gothgen VH Larsen (1995) ArticleTitleOxygen status of arterial and mixed venous blood Crit Care Med 23 1284–1293 Occurrence Handle7600839 Occurrence Handle10.1097/00003246-199507000-00020 Occurrence Handle1:STN:280:DyaK2MzisVagug%3D%3D
RH Smith EJ Guilbeau DD Reneau (1977) ArticleTitleThe oxygen tension field within a discrete volume of cerebral cortex Microvasc Res 13 233–240 Occurrence Handle875749 Occurrence Handle10.1016/0026-2862(77)90088-7 Occurrence Handle1:STN:280:DyaE2s3ht1Shtw%3D%3D
B Thony N Blau (2006) ArticleTitleMutations in the BH4-metabolizing genes GTP cyclohydrolase I, 6-pyruvoyl-tetrahydropterin synthase, sepiapterin reductase, carbinolamine-4a-dehydratase, and dihydropteridine reductase Hum Mutat 27 870–878 Occurrence Handle16917893 Occurrence Handle10.1002/humu.20366 Occurrence Handle1:CAS:528:DC%2BD28XhtFShu7nF
DE Wallick LM Bloom BJ Gaffney SJ Benkovic (1984) ArticleTitleReductive activation of phenylalanine hydroxylase and its effect on the redox state of the non-heme iron Biochemistry 23 1295–1302 Occurrence Handle6324864 Occurrence Handle10.1021/bi00301a043 Occurrence Handle1:CAS:528:DyaL2cXpvV2guw%3D%3D
Author information
Authors and Affiliations
Additional information
Authors’ address: Jan Haavik, Department of Biomedicine, University of Bergen, 5009 Bergen, Norway
Rights and permissions
About this article
Cite this article
Rostrup, M., Fossbakk, A., Hauge, A. et al. Oxygen dependence of tyrosine hydroxylase. Amino Acids 34, 455–464 (2008). https://doi.org/10.1007/s00726-007-0547-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-007-0547-7