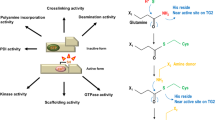
Summary.
Tissue transglutaminase catalyzes irreversible post-translational modification of specific protein substrates by either crosslinkage or incorporation of primary amines into glutamine residues, through glutamyl-amide isopeptide bonds. Modulation in vivo of these reactions (collectively called “transamidation”) is brought about by both ligand dependent effects (chiefly, activation by calcium and inhibition by GTP) as well as by variation in enzyme tissue levels by transcriptional effects. Accumulating observations that the enzyme stability in vitro is greatly affected by interaction with ligands led us to postulate that also the turn-over in vivo might be modulated by ligands opening new scenarios on the regulation of the tissue transamidating activity. This proposal is consistent with data obtained in in vitro cell culture systems and has important implications for the expression of activity in vivo.
Similar content being viewed by others
References
AM Belkin EA Zemskov J Hang SS Akimov S Sikora AY Strongin (2004) ArticleTitleCell-surface-associated tissue transglutaminase is a target of MMP-2 proteolysis Biochemistry 43 11760–11769 Occurrence Handle15362860 Occurrence Handle10.1021/bi049266z Occurrence Handle1:CAS:528:DC%2BD2cXmvVyqtL8%3D
S Beninati M Piacentini (2004) ArticleTitleThe transglutaminase family: an overview: minireview article Amino Acids 26 367–372 Occurrence Handle15290342 Occurrence Handle1:CAS:528:DC%2BD2cXmtVWktbw%3D
CM Bergamini (1988) ArticleTitleGTP modulates calcium binding and cation-induced conformational changes in erythrocyte transglutaminase FEBS Lett 239 255–258 Occurrence Handle2903073 Occurrence Handle10.1016/0014-5793(88)80928-1 Occurrence Handle1:CAS:528:DyaL1MXjsF2qtQ%3D%3D
CM Bergamini M Griffin FS Pansini (2005) ArticleTitleTransglutaminase and vascular biology: physiopathologic implications and perspectives for therapeutic interventions Curr Med Chem 12 2357–2372 Occurrence Handle16181137 Occurrence Handle10.2174/0929867054864804 Occurrence Handle1:CAS:528:DC%2BD2MXhtVWks7jP
DW Bolen IV Baskakov (2001) ArticleTitleThe osmophobic effect: natural selection of a thermodynamic force in protein folding J Mol Biol 310 955–963 Occurrence Handle11502004 Occurrence Handle10.1006/jmbi.2001.4819 Occurrence Handle1:CAS:528:DC%2BD3MXmslShtbo%3D
R Casadio E Polverini P Mariani F Spinozzi F Carsughi A Fontana P Polverino de Laureto G Matteucci CM Bergamini (1999) ArticleTitleThe structural basis for the regulation of tissue transglutaminase by calcium ions Eur J Biochem 262 672–679 Occurrence Handle10411627 Occurrence Handle10.1046/j.1432-1327.1999.00437.x Occurrence Handle1:CAS:528:DyaK1MXkt1yrtb8%3D
R Chandrashekar N Tsuji T Morales V Ozols K Mehta (1998) ArticleTitleAn ERp60-like protein from the filarial parasite Dirofilaria immitis has both transglutaminase and protein disulfide isomerase activity Proc Natl Acad Sci USA 95 531–536 Occurrence Handle9435226 Occurrence Handle10.1073/pnas.95.2.531 Occurrence Handle1:CAS:528:DyaK1cXmtFWrtQ%3D%3D
SI Chung (1975) Multiple molecular forms of transglutaminases in human and guinea pig CL Markert (Eds) Isoenzymes: molecular structure NumberInSeriesI Academic Press San Diego 259–273
A Di Venere A Rossi F De Matteis N Rosato A Finazzi-Agrò G Mei (2000) ArticleTitleOpposite effects of Ca2+ and GTP binding on tissue transglutaminase tertiary structure J Biol Chem 275 3915–3921 Occurrence Handle10660544 Occurrence Handle10.1074/jbc.275.6.3915 Occurrence Handle1:CAS:528:DC%2BD3cXht12gs70%3D
RJ Dohmen (2004) ArticleTitleSUMO protein modification Biochim Biophys Acta 1695 113–131 Occurrence Handle15571812 Occurrence Handle10.1016/j.bbamcr.2004.09.021 Occurrence Handle1:CAS:528:DC%2BD2cXhtVantbzJ
C Esposito I Caputo (2005) ArticleTitleMammalian transglutaminases. Identification of substrates as a key to physiological function and physiopathological relevance FEBS J 272 615–631 Occurrence Handle15670145 Occurrence Handle10.1111/j.1742-4658.2004.04476.x Occurrence Handle1:CAS:528:DC%2BD2MXht12htbs%3D
M Fabbi D Marimpietri S Martini C Brancolini A Amoresano A Scaloni A Bargellesi E Cosulich (1999) ArticleTitleTissue transglutaminase is a caspase substrate during apoptosis. Cleavage causes loss of transamidating function and is a biochemical marker of caspase 3 activation Cell Death Differ 6 992–1001 Occurrence Handle10556977 Occurrence Handle10.1038/sj.cdd.4400573 Occurrence Handle1:CAS:528:DyaK1MXnvFSjtLc%3D
JF Feng M Readon SP Yadav MJ Im (1999) ArticleTitleCalreticulin down-regulates both GTP binding and transglutaminase activities of transglutaminase II Biochemistry 38 10743–10749 Occurrence Handle10451369 Occurrence Handle10.1021/bi9905009 Occurrence Handle1:CAS:528:DyaK1MXks1Kjsb4%3D
BW Festoff K SantaCruz PM Arnold CT Sebastian PJ Davies BA Citron (2002) ArticleTitleInjury-induced “switch” from GTP-regulated to novel GTP-independent isoform of tissue transglutaminase in the rat spinal cord J Neurochem 81 708–718 Occurrence Handle12065630 Occurrence Handle10.1046/j.1471-4159.2002.00850.x Occurrence Handle1:CAS:528:DC%2BD38XktFCnt7k%3D
L Fesüs Z Szondy (2005) ArticleTitleTransglutaminase 2 in the balance of cell death and survival FEBS Lett 579 3297–3302 Occurrence Handle15943974 Occurrence Handle10.1016/j.febslet.2005.03.063 Occurrence Handle1:CAS:528:DC%2BD2MXltFSqu70%3D
ML Fink SI Chung JE Folk (1980) ArticleTitleγ-Glutamylamine cyclotransferase: specificity toward ɛ-(L-γ-glutamyl)-L-lysine and related compounds Proc Natl Acad Sci USA 77 4564–4568 Occurrence Handle6107907 Occurrence Handle10.1073/pnas.77.8.4564 Occurrence Handle1:CAS:528:DyaL3MXhsVOr
JE Folk MH Park SI Chung J Schrode EP Lester HL Cooper (1980) ArticleTitlePolyamines as physiological substrates for transglutaminases J Biol Chem 255 3695–3700 Occurrence Handle6102569 Occurrence Handle1:CAS:528:DyaL3cXksVShu78%3D
A Fontana (1991) ArticleTitleAnalysis and modulation of protein stability Curr Opin Biotechnol 2 551–560 Occurrence Handle1367675 Occurrence Handle10.1016/0958-1669(91)90080-O Occurrence Handle1:CAS:528:DyaK38XnvFGgsQ%3D%3D
P Grenard MK Bates D Aeschlimann (2001) ArticleTitleEvolution of transglutaminase genes: identification of a transglutaminase gene cluster on human chromosome 15q15. Structure of the gene encoding transglutaminase X and a novel gene family member, transglutaminase Z J Biol Chem 276 33066–33078 Occurrence Handle11390390 Occurrence Handle10.1074/jbc.M102553200 Occurrence Handle1:CAS:528:DC%2BD3MXmvFCmuro%3D
M Griffin R Casadio CM Bergamini (2002) ArticleTitleTransglutaminases: nature’s biological glues Biochem J 368 377–396 Occurrence Handle12366374 Occurrence Handle10.1042/BJ20021234 Occurrence Handle1:CAS:528:DC%2BD38XovF2jt78%3D
F Koning D Schuppan N Cerf-Bensussan LM Sollid (2005) ArticleTitlePathomechanisms in celiac disease Best Pract Res Clin Gastroenterol 19 373–387 Occurrence Handle15925843 Occurrence Handle10.1016/j.bpg.2005.02.003 Occurrence Handle1:CAS:528:DC%2BD2MXltFWku7o%3D
X Li P Coffino (1994) ArticleTitleDistinct domains of antizyme required for binding and proteolysis of ornithine decarboxylase Mol Cell Biol 14 87–92 Occurrence Handle8264655 Occurrence Handle1:CAS:528:DyaK2cXht1ygsL8%3D
P Mariani F Carsughi F Spinozzi S Romanzetti G Meier R Casadio CM Bergamini (2000) ArticleTitleLigand-induced conformational changes in tissue transglutaminase: Monte Carlo analysis of small-angle scattering data Biophys J 78 3240–3251 Occurrence Handle10828000 Occurrence Handle1:CAS:528:DC%2BD3cXjvFOgsL8%3D Occurrence Handle10.1016/S0006-3495(00)76860-3
S Mian S el Alaoui J Lawry V Gentile PJ Davies M Griffin (1995) ArticleTitleThe importance of the GTP-binding protein tissue transglutaminase in the regulation of cell cycle progression FEBS Lett 370 27–31 Occurrence Handle7649299 Occurrence Handle10.1016/0014-5793(95)00782-5 Occurrence Handle1:CAS:528:DyaK2MXns1Cqs70%3D
S Mishra LJ Murphy (2006) ArticleTitleThe p53 oncoprotein is a substrate for tissue transglutaminase kinase activity Biochem Biophys Res Commun 339 726–730 Occurrence Handle16313886 Occurrence Handle10.1016/j.bbrc.2005.11.071 Occurrence Handle1:CAS:528:DC%2BD2MXhtlSiurzF
L Nagy VA Thomazy MM Saydak JP Stein PJ Davies (1997) ArticleTitleThe promoter of the mouse tissue transglutaminase gene directs tissue-specific, retinoid-regulated and apoptosis-linked expression Cell Death Differ 4 534–547 Occurrence Handle14555966 Occurrence Handle10.1038/sj.cdd.4400290 Occurrence Handle1:CAS:528:DyaK2sXotVCgsrY%3D
KN Parameswaran XF Cheng EC Chen PT Velasco JH Wilson L Lorand (1997) ArticleTitleHydrolysis of gamma: epsilon isopeptides by cytosolic transglutaminases and by coagulation factor XIIIa J Biol Chem 272 10311–10317 Occurrence Handle9092583 Occurrence Handle10.1074/jbc.272.15.10311 Occurrence Handle1:CAS:528:DyaK2sXisFOks7w%3D
L Piredda MG Farrace M Lo Bello W Malorni G Melino R Petruzzelli M Piacentini (1999) ArticleTitleIdentification of ‘tissue’ transglutaminase binding proteins in neural cells committed to apoptosis FASEB J 13 355–364 Occurrence Handle9973324 Occurrence Handle1:CAS:528:DyaK1MXhtFGktbw%3D
RT Schimke (1973) ArticleTitleControl of enzyme levels in mammalian tissues Adv Enzymol Relat Areas Mol Biol 37 135–187 Occurrence Handle4570065 Occurrence Handle10.1002/9780470122822.ch3 Occurrence Handle1:STN:280:DyaE3s7jtV2nsQ%3D%3D
K Small JF Feng J Lorenz ET Donnelly A Yu MJ Im GW Dorn Suffix2nd SB Liggett (1999) ArticleTitleCardiac specific overexpression of transglutaminase II (Gh) results in a unique hypertrophy phenotype independent of phospholipase C activation J Biol Chem 274 21291–21296 Occurrence Handle10409687 Occurrence Handle10.1074/jbc.274.30.21291 Occurrence Handle1:CAS:528:DyaK1MXkvVKrtrk%3D
PA Smethurst M Griffin (1996) ArticleTitleMeasurement of tissue transglutaminase activity in a permeabilized cell system: its regulation by Ca2+ and nucleotides Biochem J 313 803–808 Occurrence Handle8611158 Occurrence Handle1:CAS:528:DyaK28XhtV2it7g%3D
V Thomazy L Fesus (1989) ArticleTitleDifferential expression of tissue transglutaminase in human cells. An immunohistochemical study Cell Tissue Res 255 215–224 Occurrence Handle2567625 Occurrence Handle10.1007/BF00229084 Occurrence Handle1:STN:280:DyaL1M3ovVahuw%3D%3D
J Zhang RP Guttmann GV Johnson (1998a) ArticleTitleTissue transglutaminase is an in situ substrate of calpain: regulation of activity J Neurochem 71 240–247 Occurrence Handle1:CAS:528:DyaK1cXktFamsbw%3D Occurrence Handle10.1046/j.1471-4159.1998.71010240.x
J Zhang M Lesort RP Guttmann GV Johnson (1998b) ArticleTitleModulation of the in situ activity of tissue transglutaminase by calcium and GTP J Biol Chem 273 2288–2295 Occurrence Handle10.1074/jbc.273.4.2288 Occurrence Handle1:CAS:528:DyaK1cXnsVKltg%3D%3D
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bergamini, C. Effects of ligands on the stability of tissue transglutaminase: studies in vitro suggest possible modulation by ligands of protein turn-over in vivo. Amino Acids 33, 415–421 (2007). https://doi.org/10.1007/s00726-006-0457-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-006-0457-0