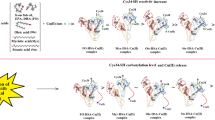
Summary.
Sulfenic acid (RSOH) is a central intermediate in both the reversible and irreversible redox modulation by reactive species of an increasing number of proteins involved in signal transduction and enzymatic pathways. In this paper we focus on human serum albumin (HSA), the most abundant plasma protein, proposed to serve antioxidant functions in the vascular compartment. Sulfenic acid in HSA has been previously detected using different methods after oxidation of its single free thiol Cys34 through one- or two-electron mechanisms. Since recent evidence suggests that sulfenic acid in HSA is stabilized within the protein environment, this derivative represents an appropriate model to examine protein sulfenic acid biochemistry, structure and reactivity. Sulfenic acid in HSA could be involved in mixed disufide formation, supporting a role of HSA-Cys34 as an important redox regulator in extracellular compartments.
Similar content being viewed by others
Abbreviations
- HSA:
-
human serum albumin
- HSA-SH:
-
the thiol of HSA
- BSA:
-
bovine serum albumin
- SH/HSA:
-
amount of thiol per albumin molecule
- RSOH:
-
sulfenic acid
- NBD-Cl:
-
7-chloro-4-nitrobenzo-2-oxa-1,3-diazol
- DTNB:
-
5,5′-dithio-bis(2-nitrobenzoic acid)
- dimedone:
-
5,5-dimethyl-1,3-cyclohexanedione
- GSH:
-
glutathione
- GSSG:
-
glutathione disulfide
References
WS Allison (1976) ArticleTitleFormation and reactions of sulfenic acids in proteins Acc Chem Res 9 293–299 Occurrence Handle10.1021/ar50104a003 Occurrence Handle1:CAS:528:DyaE2sXktl2kuw%3D%3D
B Alvarez G Ferrer-Sueta BA Freeman R Radi (1999) ArticleTitleKinetics of peroxynitrite reaction with amino acids and human serum albumin J Biol Chem 274 842–848 Occurrence Handle9873023 Occurrence Handle10.1074/jbc.274.2.842 Occurrence Handle1:CAS:528:DyaK1MXmtFKlsQ%3D%3D
A Andersson A Isaksson L Brattstrom B Hultberg (1993) ArticleTitleHomocysteine and other thiols determined in plasma by HPLC and thiol-specific postcolumn derivatization Clin Chem 39 1590–1597 Occurrence Handle8353942 Occurrence Handle1:CAS:528:DyaK3sXmt1SrsL4%3D
B Biteau J Labarre MB Toledano (2003) ArticleTitleATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin Nature 425 980–984 Occurrence Handle14586471 Occurrence Handle10.1038/nature02075 Occurrence Handle1:CAS:528:DC%2BD3sXosVOht7k%3D
R Bito S Hino A Baba M Tanaka H Watabe H Kawabata (2005) ArticleTitleDegradation of oxidative stress-induced denatured albumin in rat liver endothelial cells Am J Physiol Cell Physiol 289 C531–C542 Occurrence Handle15872008 Occurrence Handle10.1152/ajpcell.00431.2004 Occurrence Handle1:CAS:528:DC%2BD2MXhtVWksb7P
MG Bonini O Augusto (2001) ArticleTitleCarbon dioxide stimulates the production of thiyl, sulfinyl, and disulfide radical anion from thiol oxidation by peroxynitrite J Biol Chem 276 9749–9754 Occurrence Handle11134018 Occurrence Handle10.1074/jbc.M008456200 Occurrence Handle1:CAS:528:DC%2BD3MXis1OlurY%3D
S Boschi-Muller S Azza S Sanglier-Cianferani F Talfournier A Van Dorsselear G Branlant (2000) ArticleTitleA sulfenic acid enzyme intermediate is involved in the catalytic mechanism of peptide methionine sulfoxide reductase from Escherichia coli J Biol Chem 275 35908–35913 Occurrence Handle10964927 Occurrence Handle10.1074/jbc.M006137200 Occurrence Handle1:CAS:528:DC%2BD3cXosVSktL8%3D
R Brigelius-Flohe (1999) ArticleTitleTissue-specific functions of individual glutathione peroxidases Free Radic Biol Med 27 951–965 Occurrence Handle10569628 Occurrence Handle10.1016/S0891-5849(99)00173-2 Occurrence Handle1:CAS:528:DyaK1MXns1ymsrg%3D
R Bryk P Griffin C Nathan (2000) ArticleTitlePeroxynitrite reductase activity of bacterial peroxiredoxins Nature 407 211–215 Occurrence Handle11001062 Occurrence Handle10.1038/35025109 Occurrence Handle1:CAS:528:DC%2BD3cXmvVSmu74%3D
AV Budanov AA Sablina E Feinstein EV Koonin PM Chumakov (2004) ArticleTitleRegeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD Science 304 596–600 Occurrence Handle15105503 Occurrence Handle10.1126/science.1095569 Occurrence Handle1:CAS:528:DC%2BD2cXjt1Crsro%3D
S Carballal R Radi MC Kirk S Barnes BA Freeman B Alvarez (2003) ArticleTitleSulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite Biochemistry 42 9906–9914 Occurrence Handle12924939 Occurrence Handle10.1021/bi027434m Occurrence Handle1:CAS:528:DC%2BD3sXlvVyks7w%3D
DC Carter XM He (1990) ArticleTitleStructure of human serum albumin Science 249 302–303 Occurrence Handle2374930 Occurrence Handle10.1126/science.2374930 Occurrence Handle1:CAS:528:DyaK3cXlt1yhu7k%3D
DC Carter XM He SH Munson PD Twigg KM Gernert MB Broom TY Miller (1989) ArticleTitleThree-dimensional structure of human serum albumin Science 244 1195–1198 Occurrence Handle2727704 Occurrence Handle10.1126/science.2727704 Occurrence Handle1:CAS:528:DyaL1MXksFaksbY%3D
MK Cha IH Kim (2006) ArticleTitleDisulfide between Cys392 and Cys438 of human serum albumin is redox-active, which is responsible for the thioredoxin-supported lipid peroxidase activity Arch Biochem Biophys 445 19–25 Occurrence Handle16343416 Occurrence Handle1:CAS:528:DC%2BD2MXhtlGisLfE
A Claiborne TC Mallett JI Yeh J Luba D Parsonage (2001) ArticleTitleStructural, redox, and mechanistic parameters for cysteine-sulfenic acid function in catalysis and regulation Adv Protein Chem 58 215–276 Occurrence Handle11665489 Occurrence Handle1:CAS:528:DC%2BD3MXotFejsrY%3D Occurrence Handle10.1016/S0065-3233(01)58006-7
A Claiborne H Miller D Parsonage RP Ross (1993) ArticleTitleProtein-sulfenic acid stabilization and function in enzyme catalysis and gene regulation Faseb J 7 1483–1490 Occurrence Handle8262333 Occurrence Handle1:CAS:528:DyaK2cXkvVaiuw%3D%3D
A Claiborne JI Yeh TC Mallett J Luba EJ Crane Suffix3rd V Charrier D Parsonage (1999) ArticleTitleProtein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation Biochemistry 38 15407–15416 Occurrence Handle10569923 Occurrence Handle10.1021/bi992025k Occurrence Handle1:CAS:528:DyaK1MXmvFKqtbo%3D
DeLano WL (2002) The PyMOL Molecular Graphics System. DeLano Scientific, San Carlos, CA, USA. http://www.pymol.org
EG DeMaster BJ Quast B Redfern HT Nagasawa (1995) ArticleTitleReaction of nitric oxide with the free sulfhydryl group of human serum albumin yields a sulfenic acid and nitrous oxide Biochemistry 34 11494–11499 Occurrence Handle7547878 Occurrence Handle10.1021/bi00036a023 Occurrence Handle1:CAS:528:DyaK2MXns1Cisrw%3D
JM Denu KG Tanner (1998) ArticleTitleSpecific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation Biochemistry 37 5633–5642 Occurrence Handle9548949 Occurrence Handle10.1021/bi973035t Occurrence Handle1:CAS:528:DyaK1cXislGht7k%3D
P Di Simplicio S Frosali R Priora D Summa F Cherubini Di Simplicio D Di Giuseppe A Di Stefano (2005) ArticleTitleBiochemical and biological aspects of protein thiolation in cells and plasma Antioxid Redox Signal 7 951–963 Occurrence Handle15998250 Occurrence Handle10.1089/ars.2005.7.951 Occurrence Handle1:CAS:528:DC%2BD2MXlsl2isb4%3D
HR Ellis LB Poole (1997a) ArticleTitleNovel application of 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole to identify cysteine sulfenic acid in the AhpC component of alkyl hydroperoxide reductase Biochemistry 36 15013–15018 Occurrence Handle10.1021/bi972191x Occurrence Handle1:CAS:528:DyaK2sXnt1SlsL8%3D
HR Ellis LB Poole (1997b) ArticleTitleRoles for the two cysteine residues of AhpC in catalysis of peroxide reduction by alkyl hydroperoxide reductase from Salmonella typhimurium Biochemistry 36 13349–13356 Occurrence Handle10.1021/bi9713658 Occurrence Handle1:CAS:528:DyaK2sXmsVemsbc%3D
S Era T Hamaguchi M Sogami K Kuwata E Suzuki K Miura K Kawai Y Kitazawa H Okabe A Noma et al. (1988) ArticleTitleFurther studies on the resolution of human mercapt- and nonmercaptalbumin and on human serum albumin in the elderly by high-performance liquid chromatography Int J Pept Protein Res 31 435–442 Occurrence Handle3410634 Occurrence Handle1:CAS:528:DyaL1cXktlCltLw%3D Occurrence Handle10.1111/j.1399-3011.1988.tb00900.x
S Era K Kuwata H Imai K Nakamura T Hayashi M Sogami (1995) ArticleTitleAge-related change in redox state of human serum albumin Biochim Biophys Acta 1247 12–16 Occurrence Handle7873580
T Finkel (2000) ArticleTitleRedox-dependent signal transduction FEBS Lett 476 52–54 Occurrence Handle10878249 Occurrence Handle10.1016/S0014-5793(00)01669-0 Occurrence Handle1:CAS:528:DC%2BD3cXktlKqsbo%3D
RM Gatti R Radi O Augusto (1994) ArticleTitlePeroxynitrite-mediated oxidation of albumin to the protein-thiyl free radical FEBS Lett 348 287–290 Occurrence Handle8034056 Occurrence Handle10.1016/0014-5793(94)00625-3 Occurrence Handle1:CAS:528:DyaK2cXlslegtrg%3D
GI Giles C Jacob (2002) ArticleTitleReactive sulfur species: an emerging concept in oxidative stress Biol Chem 383 375–388 Occurrence Handle12033429 Occurrence Handle10.1515/BC.2002.042 Occurrence Handle1:CAS:528:DC%2BD38XksVajtrg%3D
B Halliwell (1988) ArticleTitleAlbumin an important extracellular antioxidant? Biochem Pharmacol 37 569–571 Occurrence Handle3277637 Occurrence Handle10.1016/0006-2952(88)90126-8 Occurrence Handle1:CAS:528:DyaL1cXhsVOmtbo%3D
B Halliwell JM Gutteridge (1990) ArticleTitleThe antioxidants of human extracellular fluids Arch Biochem Biophys 280 1–8 Occurrence Handle2191627 Occurrence Handle10.1016/0003-9861(90)90510-6 Occurrence Handle1:CAS:528:DyaK3cXkt1Whs7w%3D
JM Hansen YM Go DP Jones (2006a) ArticleTitleNuclear and mitochondrial compartmentation of oxidative stress and redox signaling Annu Rev Pharmacol Toxicol 46 215–234 Occurrence Handle10.1146/annurev.pharmtox.46.120604.141122 Occurrence Handle1:CAS:528:DC%2BD28XisFKltb4%3D
JM Hansen H Zhang DP Jones (2006b) ArticleTitleDifferential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions Free Radic Biol Med 40 138–145 Occurrence Handle10.1016/j.freeradbiomed.2005.09.023 Occurrence Handle1:CAS:528:DC%2BD2MXhtlSiu73J
XM He DC Carter (1992) ArticleTitleAtomic structure and chemistry of human serum albumin Nature 358 209–215 Occurrence Handle1630489 Occurrence Handle10.1038/358209a0 Occurrence Handle1:CAS:528:DyaK38XlsVGmurg%3D
H Imai T Hayashi T Negawa K Nakamura M Tomida K Koda T Tajima Y Koda K Suda S Era (2002) ArticleTitleStrenuous exercise-induced change in redox state of human serum albumin during intensive kendo training Jpn J Physiol 52 135–140 Occurrence Handle12139771 Occurrence Handle10.2170/jjphysiol.52.135 Occurrence Handle1:CAS:528:DC%2BD38Xmt1ait74%3D
T Inayama Y Kumagai M Sakane M Saito M Matsuda (1996) ArticleTitlePlasma protein-bound sulfhydryl group oxidation in humans following a full marathon race Life Sci 59 573–578 Occurrence Handle8761346 Occurrence Handle10.1016/0024-3205(96)00338-4 Occurrence Handle1:CAS:528:DyaK28XksFSks7k%3D
J Janatova JK Fuller MJ Hunter (1968) ArticleTitleThe heterogeneity of bovine albumin with respect to sulfhydryl and dimer content J Biol Chem 243 3612–3622 Occurrence Handle5690602 Occurrence Handle1:CAS:528:DyaF1cXkt1Cmu7k%3D
K Kawai M Yoh T Hayashi H Imai T Negawa M Tomida M Sogami S Era (2001) ArticleTitleEffect of diabetic retinopathy on redox state of aqueous humor and serum albumin in patients with senile cataract Tokai J Exp Clin Med 26 93–99 Occurrence Handle11885755 Occurrence Handle1:CAS:528:DC%2BD38XislOjsbw%3D
DA Keire E Strauss W Guo B Noszal DL Rabenstein (1992) ArticleTitleKinetics and equilibria of thiol/disulfide interchange reactions of selected biological thiols and related molecules with oxidized glutathione J Org Chem 57 123–127 Occurrence Handle10.1021/jo00027a023 Occurrence Handle1:CAS:528:DyaK38Xjs1Gltw%3D%3D
JL Kice JP Cleveland (1973) ArticleTitleNucleophilic substitution reactions involving sulfenic acids and sulfenyl derivatives. Nucleophile- and acid-catalyzed oxygen-18 exchange of phenyl benzenethiolsulfinate J Amer Chem Soc 95 104–109 Occurrence Handle10.1021/ja00782a017 Occurrence Handle1:CAS:528:DyaE3sXntFShtg%3D%3D
WH Koppenol JJ Moreno WA Pryor H Ischiropoulos JS Beckman (1992) ArticleTitlePeroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide Chem Res Toxicol 5 834–842 Occurrence Handle1336991 Occurrence Handle10.1021/tx00030a017 Occurrence Handle1:CAS:528:DyaK38Xmt1Oqs7w%3D
NA Kratochwil AI Ivanov M Patriarca JA Parkinson AM Gouldsworthy P Murdoch PJ Sadler (1999) ArticleTitleSurprising reactions of iodo Pt(IV) and Pt(II) complexes with human albumin: detection of Cys34 sulfenic acid J Am Chem Soc 121 8193–8203 Occurrence Handle10.1021/ja990768n Occurrence Handle1:CAS:528:DyaK1MXltlans74%3D
S Leto MJ Yiengst CH Barrows SuffixJr (1970) ArticleTitleThe effect of age and protein deprivation on the sulfhydryl content of serum albumin J Gerontol 25 4–8 Occurrence Handle5435994 Occurrence Handle1:CAS:528:DyaE3cXktVWmtL0%3D
L Liu A Hausladen M Zeng L Que J Heitman JS Stamler (2001) ArticleTitleA metabolic enzyme for S-nitrosothiol conserved from bacteria to humans Nature 410 490–494 Occurrence Handle11260719 Occurrence Handle10.1038/35068596 Occurrence Handle1:CAS:528:DC%2BD3MXisFahsbY%3D
MA Mansoor AM Svardal PM Ueland (1992) ArticleTitleDetermination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine, and glutathione in human plasma Anal Biochem 200 218–229 Occurrence Handle1632485 Occurrence Handle10.1016/0003-2697(92)90456-H Occurrence Handle1:CAS:528:DyaK38XhtVajsrw%3D
SE Moriarty-Craige DP Jones (2004) ArticleTitleExtracellular thiols and thiol/disulfide redox in metabolism Annu Rev Nutr 24 481–509 Occurrence Handle15189129 Occurrence Handle10.1146/annurev.nutr.24.012003.132208 Occurrence Handle1:CAS:528:DC%2BD2cXlvFKksLw%3D
JK Noel MJ Hunter (1972) ArticleTitleBovine mercaptalbumin and non-mercaptalbumin monomers. Interconversions and structural differences J Biol Chem 247 7391–7406 Occurrence Handle4638551 Occurrence Handle1:STN:280:DyaE3s%2Fltl2isA%3D%3D
MS Paget MJ Buttner (2003) ArticleTitleThiol-based regulatory switches Annu Rev Genet 37 91–121 Occurrence Handle14616057 Occurrence Handle10.1146/annurev.genet.37.110801.142538 Occurrence Handle1:CAS:528:DC%2BD2cXhsFGltA%3D%3D
IV Peshenko H Shichi (2001) ArticleTitleOxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite Free Radic Biol Med 31 292–303 Occurrence Handle11461766 Occurrence Handle10.1016/S0891-5849(01)00579-2 Occurrence Handle1:CAS:528:DC%2BD3MXlt1Smsbs%3D
T Peters (1996) All about albumin: biochemistry, genetics and medical applications Academic Press San Diego
LB Poole BB Zeng SA Knaggs M Yakubu SB King (2005) ArticleTitleSynthesis of chemical probes to map sulfenic acid modifications on proteins Bioconjug Chem 16 1624–1628 Occurrence Handle16287263 Occurrence Handle10.1021/bc050257s Occurrence Handle1:CAS:528:DC%2BD2MXhtFKhtbjO
C Quijano B Alvarez RM Gatti O Augusto R Radi (1997) ArticleTitlePathways of peroxynitrite oxidation of thiol groups Biochem J 322 167–173 Occurrence Handle9078258 Occurrence Handle1:CAS:528:DyaK2sXhslKruro%3D
R Radi JS Beckman KM Bush BA Freeman (1991a) ArticleTitlePeroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide J Biol Chem 266 4244–4250 Occurrence Handle1:CAS:528:DyaK3MXhsFyku7Y%3D
R Radi KM Bush TP Cosgrove BA Freeman (1991b) ArticleTitleReaction of xanthine oxidase-derived oxidants with lipid and protein of human plasma Arch Biochem Biophys 286 117–125 Occurrence Handle10.1016/0003-9861(91)90016-C Occurrence Handle1:CAS:528:DyaK3MXhsVSqtbY%3D
MJ Raftery Z Yang SM Valenzuela CL Geczy (2001) ArticleTitleNovel intra- and inter-molecular sulfinamide bonds in S100A8 produced by hypochlorite oxidation J Biol Chem 276 33393–33401 Occurrence Handle11445563 Occurrence Handle10.1074/jbc.M101566200 Occurrence Handle1:CAS:528:DC%2BD3MXmslymu7o%3D
R Sahoo T Dutta A Das S Sinha Ray R Sengupta S Ghosh (2006) ArticleTitleEffect of nitrosative stress on Schizosaccharomyces pombe: inactivation of glutathione reductase by peroxynitrite Free Radic Biol Med 40 625–631 Occurrence Handle16458193 Occurrence Handle10.1016/j.freeradbiomed.2005.09.029 Occurrence Handle1:CAS:528:DC%2BD28XhtFarsr4%3D
A Salmeen JN Andersen MP Myers TC Meng JA Hinks NK Tonks D Barford (2003) ArticleTitleRedox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate Nature 423 769–773 Occurrence Handle12802338 Occurrence Handle10.1038/nature01680 Occurrence Handle1:CAS:528:DC%2BD3sXksV2itrc%3D
A Salmeen D Barford (2005) ArticleTitleFunctions and mechanisms of redox regulation of cysteine-based phosphatases Antioxid Redox Signal 7 560–577 Occurrence Handle15890001 Occurrence Handle10.1089/ars.2005.7.560 Occurrence Handle1:CAS:528:DC%2BD2MXktVWisb4%3D
AT Saurin H Neubert JP Brennan P Eaton (2004) ArticleTitleWidespread sulfenic acid formation in tissues in response to hydrogen peroxide Proc Natl Acad Sci USA 101 17982–17987 Occurrence Handle15604151 Occurrence Handle10.1073/pnas.0404762101 Occurrence Handle1:CAS:528:DC%2BD2MXjsl2gtw%3D%3D
FQ Schafer GR Buettner (2001) ArticleTitleRedox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple Free Radic Biol Med 30 1191–1212 Occurrence Handle11368918 Occurrence Handle10.1016/S0891-5849(01)00480-4 Occurrence Handle1:CAS:528:DC%2BD3MXjsFegt78%3D
JE Schnitzer A Sung R Horvat J Bravo (1992) ArticleTitlePreferential interaction of albumin-binding proteins, gp30 and gp18, with conformationally modified albumins. Presence in many cells and tissues with a possible role in catabolism J Biol Chem 267 24544–24553 Occurrence Handle1447200 Occurrence Handle1:CAS:528:DyaK38XmtV2ntrg%3D
JS Stamler DI Simon JA Osborne ME Mullins O Jaraki T Michel DJ Singel J Loscalzo (1992) ArticleTitleS-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds Proc Natl Acad Sci USA 89 444–448 Occurrence Handle1346070 Occurrence Handle10.1073/pnas.89.1.444 Occurrence Handle1:CAS:528:DyaK38XotFWqsg%3D%3D
AJ Stewart CA Blindauer S Berezenko D Sleep D Tooth PJ Sadler (2005) ArticleTitleRole of Tyr84 in controlling the reactivity of Cys34 of human albumin Febs J 272 353–362 Occurrence Handle15654874 Occurrence Handle10.1111/j.1742-4658.2004.04474.x Occurrence Handle1:CAS:528:DC%2BD2MXptFejtA%3D%3D
S Sugio A Kashima S Mochizuki M Noda K Kobayashi (1999) ArticleTitleCrystal structure of human serum albumin at 2.5 A resolution Protein Eng 12 439–446 Occurrence Handle10388840 Occurrence Handle10.1093/protein/12.6.439 Occurrence Handle1:CAS:528:DyaK1MXkslGhsb4%3D
PS Surdhar DA Armstrong (1986) ArticleTitleRedox potentials of some sulfur-containing radicals J Phys Chem 90 5915–5917 Occurrence Handle10.1021/j100280a091 Occurrence Handle1:CAS:528:DyaL28Xlsl2gtL8%3D
K Takakura JS Beckman LA MacMillan-Crow JP Crow (1999) ArticleTitleRapid and irreversible inactivation of protein tyrosine phosphatases PTP1B, CD45, and LAR by peroxynitrite Arch Biochem Biophys 369 197–207 Occurrence Handle10486138 Occurrence Handle10.1006/abbi.1999.1374 Occurrence Handle1:CAS:528:DyaK1MXlvVWrtb8%3D
M Tomida J Ishimaru T Hayashi K Nakamura K Murayama S Era (2003) ArticleTitleThe redox states of serum and synovial fluid of patients with temporomandibular joint disorders Jpn J Physiol 53 351–355 Occurrence Handle14975181 Occurrence Handle10.2170/jjphysiol.53.351
YM Torchinsky (1981) Sulfur in proteins Pergamon Press Ltd Oxford
M Trujillo H Budde MD Pineyro M Stehr C Robello L Flohe R Radi (2004) ArticleTitleTrypanosoma brucei and Trypanosoma cruzi tryparedoxin peroxidases catalytically detoxify peroxynitrite via oxidation of fast reacting thiols J Biol Chem 279 34175–34182 Occurrence Handle15155760 Occurrence Handle10.1074/jbc.M404317200 Occurrence Handle1:CAS:528:DC%2BD2cXmvFent74%3D
M Trujillo R Radi (2002) ArticleTitlePeroxynitrite reaction with the reduced and the oxidized forms of lipoic acid: new insights into the reaction of peroxynitrite with thiols Arch Biochem Biophys 397 91–98 Occurrence Handle11747314 Occurrence Handle10.1006/abbi.2001.2619 Occurrence Handle1:CAS:528:DC%2BD3MXptFWrtr8%3D
A van der Vliet PA Hoen PS Wong A Bast CE Cross (1998) ArticleTitleFormation of S-nitrosothiols via direct nucleophilic nitrosation of thiols by peroxynitrite with elimination of hydrogen peroxide J Biol Chem 273 30255–30262 Occurrence Handle9804785 Occurrence Handle10.1074/jbc.273.46.30255 Occurrence Handle1:CAS:528:DyaK1cXnsVyrtLw%3D
RL van Montfort M Congreve D Tisi R Carr H Jhoti (2003) ArticleTitleOxidation state of the active-site cysteine in protein tyrosine phosphatase 1B Nature 423 773–777 Occurrence Handle12802339 Occurrence Handle10.1038/nature01681 Occurrence Handle1:CAS:528:DC%2BD3sXksV2it74%3D
JM Wilson D Wu R Motiu-DeGrood DJ Hupe (1980) ArticleTitleA spectrophotometric method for studying the rates of reaction of disulfides with protein thiol groups applied to bovine serum albumin J Am Chem Soc 102 359–363 Occurrence Handle10.1021/ja00521a058 Occurrence Handle1:CAS:528:DyaL3cXntFCjsw%3D%3D
DA Wink RW Nims JF Darbyshire D Christodoulou I Hanbauer GW Cox F Laval J Laval JA Cook MC Krishna et al. (1994) ArticleTitleReaction kinetics for nitrosation of cysteine and glutathione in aerobic nitric oxide solutions at neutral pH. Insights into the fate and physiological effects of intermediates generated in the NO/O2 reaction Chem Res Toxicol 7 519–525 Occurrence Handle7981416 Occurrence Handle10.1021/tx00040a007 Occurrence Handle1:CAS:528:DyaK2cXksFWrs70%3D
CC Winterbourn D Metodiewa (1999) ArticleTitleReactivity of biologically important thiol compounds with superoxide and hydrogen peroxide Free Radic Biol Med 27 322–328 Occurrence Handle10468205 Occurrence Handle10.1016/S0891-5849(99)00051-9 Occurrence Handle1:CAS:528:DyaK1MXltlKrsL8%3D
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carballal, S., Alvarez, B., Turell, L. et al. Sulfenic acid in human serum albumin. Amino Acids 32, 543–551 (2007). https://doi.org/10.1007/s00726-006-0430-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-006-0430-y