Summary.
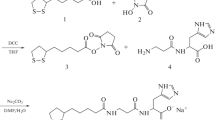
Three novel carnosine analogues 7–9 containing the residue of L(+)2,3-diaminopropionic acid with different degree of N-acetylation instead of β-alanine have been synthesized and characterized. Comparative analysis of hydrolysis by carnosinase revealed that the mono- and bis-acetylated compounds 8 and 9 are resistant to enzymatic hydrolysis and act as competitive inhibitors of this enzyme. The hydroxyl radical scavenging potential of the three analogues was evaluated by their ability to inhibit iron/H2O2-induced degradation of deoxyribose. The second-order rate constants of the reaction of compounds 7–9 with hydroxyl radical were almost identical to that of carnosine. These compounds were also found to act as protective agents against peroxynitrite-dependent damage as assessed by their ability to prevent nitration of free tyrosine induced by this species.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cacciatore, I., Cocco, A., Costa, M. et al. Biochemical properties of new synthetic carnosine analogues containing the residue of 2,3-diaminopropionic acid: the effect of N-acetylation. Amino Acids 28, 77–83 (2005). https://doi.org/10.1007/s00726-004-0142-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-004-0142-0