Abstract
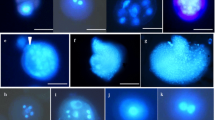
Production of doubled haploid (DH) plants is an efficient tool in genetic and plant breeding programs; however, sweet pepper (Capsicum annuum L.) is recalcitrant to microspore embryogenesis and DH production. Trying to break the barrier of DH production, three independent experiments were conducted on microspore embryogenesis of sweet pepper. In the first experiment, the effect of cold (4 °C) and heat (32 °C) pretreatments were investigated on microspore embryogenesis of three genotypes of sweet pepper including “Inspiration F1,” “Maratus F1,” and “Magno F1” cultivars in a factorial design with three replications. Heat shock (32 °C for 7 days), applied to mannitol-starved anthers of “Inspiration F1,” showed higher multinuclear microspore percent, number of multicellular structures, total embryos, cotyledonary embryos, and regenerants. In the second experiment, the effect of different concentrations of putrescine (0, 0.5, 1, 2, and 5 mg l−1) was evaluated on microspore embryogenesis of the three aforementioned cultivars of sweet pepper. The highest mean number of multicellular structures, cotyledonary embryos, and regenerants were achieved by applying 0.5–1 mg l−1 putrescine during the mannitol starvation and heat shock (32 °C) treatments of isolated microspore culture of “Inspiration F1” cultivar. Significant decrease in microspore embryogenesis efficiency was observed when high levels of putrescine (2 and 5 mg l−1) were used. Microspore embryogenesis was prevented completely at 5.0 mg l−1 putrescine. In the third experiment, the effect of different concentrations of ascorbic acid (0, 20, 50, 100, and 200 mg l−1) was investigated and the results showed that the application of ascorbic acid (20 and 50 mg l−1) during mannitol starvation and heat shock treatment (32 °C) caused remarkable improvement in the number of produced cotyledonary embryos and their regeneration ability compared to control treatment. However, the application of higher levels of ascorbic acid (100 and 200 mg l−1) inhibited microspore cell divisions and embryogenesis. In conclusion, the results indicated that both putrescine and ascorbic acid have significant effect on microspore embryogenesis efficiency of sweet pepper when they are used in appropriate concentrations.



Similar content being viewed by others
Abbreviations
- AA:
-
Ascorbic acid
- ACC:
-
1-aminocyclopropane-1-carboxylic acid
- CE:
-
Cotyledonary embryo
- DH:
-
Doubled haploid
- MCS:
-
Multicellular structure
- MNM:
-
Multinuclear microspores
- ROS:
-
Reactive oxygen species
- TE:
-
Total embryo
- CRD:
-
Completely randomized design
References
Ahmadi B, Shariatpanahi ME, Ojaghkandi MA, Heydari AA (2014) Improved microspore embryogenesis induction and plantlet regeneration using putrescine, cefotaxime and vancomycin in Brassica napus L. Plant Cell Tissue Organ Cult 118:497–505. https://doi.org/10.1007/s11240-014-0501-9
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249. https://doi.org/10.1007/s00425-010-1130-0
Altman A (1989) Polyamines and plant hormones. Physiol Polyam 2:121–145
Arrigoni O, Calabrese G, Degara L et al (1997) Correlation between changes in cell ascorbate and growth of Lupinus albus seedlings. J Plant Physiol 150:302–308. https://doi.org/10.1016/S0176-1617(97)80125-3
Arrigoni O, De Tullio MC (2000) The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol 157:481–488. https://doi.org/10.1016/S0176-1617(00)80102-9
Avilés-Viñas SA, Lecona-Guzmán CA, Canto-Flick A, López-Erosa S, Santana-Buzzy N (2013) Morpho-histological and ultrastructural study on direct somatic embryogenesis of Capsicum chinense Jacq. in liquid medium. Plant Biotechnol Rep 7:277–286. https://doi.org/10.1007/s11816-012-0261-0
Bajaj YPS (1990) In vitro production of haploids and their use in cell genetics and plant breeding. In: Bajaj YPS (ed) Biotechnol Agric For, vol. 12. Haploids in crop improvement. Springer-Verlag Berlin Heidelberg, Berlin, pp 3–49
Biddington NL, Robinson HT (1991) Ethylene production during anther culture of Brussels sprouts (Brassica oleracea var gemmifera) and its relationship with factors that affect embryo production. Plant Cell Tissue Organ Cult 25:169–177. https://doi.org/10.1007/BF00036207
Chen Z, Gallie DR (2012) Induction of monozygotic twinning by ascorbic acid in tobacco. PLoS One 7:e39147
Christensen HM, Bamford R (1943) Haploids in twin seedlings of pepper: Capsicum annuum L| _. J Hered 34:99–104
Custers JBM (2003) Microspore culture in rapeseed (Brassica napus L.). In: Doubled haploid production in crop plants. Springer, pp 185–193
De Cabo RC, González-Reyes JA, Cordoba F, Navas P (1996) Rooting hastened in onions by ascorbate and ascorbate free radical. J Plant Growth Regul 15:53–56
De Tullio MC, Asard H, May JM, Smirnoff N (2002) Vitamin C function and biochemistry in animals and plants. Garland Science, Oxon 323 p
Dewi IS, Purwoko BS (2008) Role of polyamines in inhibition of ethylene biosynthesis and their effects on rice anther. Indones J Agric Sci 9:60–67
Dpooležel J, Binarová P, Lcretti S (1989) Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant 31:113–120
Dumas de Vaulx R, Chambonnet D, Pochard E (1981) In vitro culture of pepper (Capsicum annuum L.) anthers: high rate plant production from different genotypes by+ 35 degrees C treatments [haploidy, temperature]. Agronomie (France)
Ebrahimzadeh H, Shariatpanahi ME, Ahmadi B, Soltanloo H, Lotfi M, Zarifi E (2018) Efficient parthenogenesis induction and in vitro haploid plant regeneration in cucumber (Cucumis sativus L.) using putrescine, spermidine, and cycocel. J Plant Growth Regul. https://doi.org/10.1007/s00344-018-9803-1
Ferrie a MR, Caswell KL (2011) Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell Tissue Organ Cult 104:301–309. https://doi.org/10.1007/s11240-010-9800-y
Foyer CH (2003) The role of ascorbic acid in defense networks and signalling in plants. Vitam C its Funct Biochem Anim Plants 65–82
Fry SC (1988) The growing plant cell wall: chemical and metabolic analysis. New York 203:333. https://doi.org/10.1111/j.1469-8137.2003.00980.x
Galston AW, Kaur-Sawhney R, Altabella T, Tiburcio AF (1997) Plant polyamines in reproductive activity and response to abiotic stress. Plant Biol 110:197–207
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. https://doi.org/10.1016/0014-4827(68)90403-5
Gémes-Juhász a, Balogh P, Ferenczy a, Kristóf Z (2002) Effect of optimal stage of female gametophyte and heat treatment on in vitro gynogenesis induction in cucumber (Cucumis sativus L.). Plant Cell Rep 21:105–111. https://doi.org/10.1007/s00299-002-0482-8
Habibi N, Suthar RK, Purohit SD (2009) Role of PGrs and inhibitors in induction and control of somatic embryogenesis in Themeda quadrivalvis. Indian J Exp Biol 47:198–203
Heidmann I, de Lange B, Lambalk J, Angenent GC, Boutilier K (2011) Efficient sweet pepper transformation mediated by the BABY BOOM transcription factor. Plant Cell Rep 30:1107–1115. https://doi.org/10.1007/s00299-011-1018-x
Hoseini M, Ghadimzadeh M, Ahmadi B, da Silva JAT (2014) Effects of ascorbic acid, alpha-tocopherol, and glutathione on microspore embryogenesis in Brassica napus L. Vitr Cell Dev Biol 50:26–35
Kasha KJ, Maluszynski M (2003) Production of doubled haploids in crop plants. An introduction. In: Doubled haploid production in crop plants. Springer, pp 1–4
Kaur-sawhney R, Tiburcio AF, Altabella T, Galston AW (2003) Polyamines in plants: an overview. J Cell Mol Biol 2:1–12
Kelley RY, Zipf AE, Wesenberg DE, Sharma GC (2002) Putrescine-enhanced somatic embryos and plant numbers from elite oat (Avena spp. L.) and reciprocal crosses. In Vitro Cellular & Developmental Biology-Plant 38(5):508–512
Kevers C, Gaspar T, Dommes J (2002) The beneficial role of different auxins and polyamines at successive stages of somatic embryo formation and development of Panax ginseng in vitro. Plant Cell Tissue Organ Cult 70(2):181–188
Kim M, Jang I-C, Kim J-A, Park EJ, Yoon M, Lee Y (2008) Embryogenesis and plant regeneration of hot pepper (Capsicum annuum L.) through isolated microspore culture. Plant Cell Rep 27:425–434. https://doi.org/10.1007/s00299-007-0442-4
Kothari SL, Joshi A, Kachhwaha S, Ochoa-Alejo N (2010) Chilli peppers—a review on tissue culture and transgenesis. Biotechnol Adv 28:35–48. https://doi.org/10.1016/j.biotechadv.2009.08.005
Kumar HGA, Ravishankar BV, Murthy HN (2004) The influence of polyamines on androgenesis of Cucumis sativus L. Eur J Hortic Sci:201–205
Lantos C, Juhász AG, Somogyi G, Ötvös K, Vági P, Mihály R, Kristóf Z, Somogyi N, Pauk J (2009) Improvement of isolated microspore culture of pepper (Capsicum annuum L.) via co-culture with ovary tissues of pepper or wheat. Plant Cell Tissue Organ Cult 97:285–293. https://doi.org/10.1007/s11240-009-9527-9
Lantos C, Juhász AG, Vági P et al (2012) Androgenesis induction in microspore culture of sweet pepper (Capsicum annuum L.). Plant Biotechnol Rep 6:123–132. https://doi.org/10.1007/s11816-011-0205-0
Leroux B, Carmoy N, Giraudet D, Potin P, Larher F, Bodin M (2009) Inhibition of ethylene biosynthesis enhances embryogenesis of cultured microspores of Brassica napus. Plant Biotechnol Rep 3:347–353. https://doi.org/10.1007/s11816-009-0109-4
Liso R, Innocenti AM, Bitonti MB, Arrigoni O (1988) Ascorbic acid-induced progression of quiescent centre cells from G1 to S phase. New Phytol 110:469–471
Liszkay A, Kenk B, Schopfer P (2003) Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 217:658–667. https://doi.org/10.1007/s00425-003-1028-1
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Mityko J, Andrasfalvy A, Csillery G, Fari M (1995) Anther-culture response in different genotypes and Fi hyhrids of pepper (Capsicum annuum L.). 80:78–81
Navas P, Gomezdiaz C (1995) Ascorbate free radical and its role in growth control. Protoplasma 184:8–13
Nichol JW, Slade D, Viss P, Stuart DA (1991) Effect of organic acid pretreatment on the regeneration and development (conversion) of whole plants from callus cultures of alfalfa, Medicago sativa L. Plant Sci 79:181–192. https://doi.org/10.1016/0168-9452(91)90105-H
Pal Bais H, Ravishankar GA (2002) Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tissue Organ Cult 69:1–34. https://doi.org/10.1023/A:1015064227278
Pauling L (1971) Vitamin C and the common cold. Can Med Assoc J 105(448):450
Pickersgill B (1997) Genetic resources and breeding of Capsicum spp. Euphytica 96:129–133. https://doi.org/10.1023/A:1002913228101
Potters G, De Gara L, Asard H, Horemans N (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40:537–548. https://doi.org/10.1016/S0981-9428(02)01414-6
Potters G, Horemans N, Bellone S, Caubergs RJ, Trost P, Guisez Y, Asard H (2004) Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiol 134:1479–1487. https://doi.org/10.1104/pp.103.033548
Pourabdollah Najafabadi F, Enayati Shariatpanahi M, Ahmadi B, Sima NKK, Alizadeh B, Oroojloo M (2015) Effects of heat shock and 2, 4-D treatment on morphological and physiological characteristics of microspores and microspore-derived doubled haploid plants in Brassica napus L. Iran J Biotechnol 13:31–38. https://doi.org/10.15171/ijb.1148
Redha A, Suleman P (2011) Effects of exogenous application of polyamines on wheat anther cultures. Plant Cell, Tissue and Organ Culture (PCTOC) 105(3):345–353
Regla-Márquez CF, Canto-Flick A, Avilés-Viñas SA, Valle-Gough RE, Pérez-Pastrana J, García-Villalobos FJ, Santana-Buzzy N (2016) Cadaverine: a common polyamine in zygotic embryos and somatic embryos of the species Capsicum chinense Jacq. Plant Cell Tissue Organ Cult 124:253–264. https://doi.org/10.1007/s11240-015-0889-x
Rodríguez-Serrano M, Bárány I, Prem D et al (2012) NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J Exp Bot 63:2007–2024. https://doi.org/10.1093/jxb/err400
Sakhanokho HF, Ozias-Akins P, May OL, Chee PW (2005) Putrescine enhances somatic embryogenesis and plant regeneration in upland cotton. Plant Cell Tissue Organ Cult 81:91–95. https://doi.org/10.1007/s11240-004-1541-3
Satpute GK, Long H, Seguí-Simarro JM, Risueño MC, Testillano PS (2005) Cell architecture during gametophytic and embryogenic microspore development in Brassica napus L. Acta Physiol Plant 27:665–674
Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A (2002) Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214:821–828. https://doi.org/10.1007/s00425-001-0699-8
Seguí-Simarro JM (2016) Androgenesis in solanaceae. Vitr Embryog High Plants:209–244
Shariatpanahi ME, Bal U, Heberle-Bors E, Touraev A (2006) Stresses applied for the re-programming of plant microspores towards in vitro embryogenesis. Physiol Plant 127:519–534. https://doi.org/10.1111/j.1399-3054.2006.00675.x
Shariatpanahi ME, Ahmadi B (2016) Isolated microspore culture and its applications in plant breeding and genetics. In: Plant tissue culture: propagation, conservation and crop improvement. Springer, Singapore, pp 487–507
Sibi ML, Kobaissi A, Shekafandeh A (2001) Green haploid plants from unpollinated ovary culture in tetraploid wheat (Triticum durum Defs.). Euphytica 122:351–359
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 35:291–314. https://doi.org/10.1080/07352680091139231
Smith JJ, Ververidis P, John P (1992) Characterization of the ethylene-forming enzyme partially purified from melon. Phytochemistry 31:1485–1494
Steinitz B, Küsek M, Tabib Y, Paran I, Zelcer A (2003) Pepper (Capsicum annuum L.) regenerants obtained by direct somatic embryogenesis fail to develop a shoot. Vitr Cell Dev Biol - Plant 39:296–303. https://doi.org/10.1079/IVP2002405
Supena EDJ, Custers JBM (2011) Refinement of shed-microspore culture protocol to increase normal embryos production in hot pepper (Capsicum annuum L.). Sci Hortic (Amsterdam) 130:769–774. https://doi.org/10.1016/j.scienta.2011.08.037
Supena EDJ, Suharsono S, Jacobsen E, Custers JBM (2006) Successful development of a shed-microspore culture protocol for doubled haploid production in Indonesian hot pepper (Capsicum annuum L.). Plant Cell Rep 25:1–10. https://doi.org/10.1007/s00299-005-0028-y
Tiainen T (1992) The role of ethylene and reducing agents on anther culture response of tetraploid potato (Solanum tuberosum L.). Plant Cell Rep 10:604–607
Tokunaga T, Miyahara K, Tabata K, Esaka M (2005) Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for l-galactono-1,4-lactone dehydrogenase. Planta 220:854–863. https://doi.org/10.1007/s00425-004-1406-3
Touraev A, Ilham A, Vicente O, Heberle-Bors E (1996a) Stress-induced microspore embryogenesis in tobacco: an optimized system for molecular studies. Plant Cell Rep 15:561–565. https://doi.org/10.1007/BF00232453
Touraev A, Indrianto A, Wratschko I, Vicente O, Heberle-Bors E (1996b) Efficient microspore embryogenesis in wheat (Triticum aestivum L.) induced by starvation at high temperature. Sex Plant Reprod 9:209–215. https://doi.org/10.1007/s004970050033
Us-Camas R, Rivera-Solís G, Duarte-Aké F, De-la-Peña C (2014) In vitro culture: an epigenetic challenge for plants. Plant Cell Tissue Organ Cult 118:187–201. https://doi.org/10.1007/s11240-014-0482-8
Acknowledgements
The authors would like to express their deepest appreciation to Dr. Mohsen Niazian and Dr. Maryam Jafarkhani-Kermani for their excellent manuscript editorial helps and critical reading. This research was supported by grants from Agricultural Biotechnology Research Institute of Iran (ABRII) Project No. 14-05-05-9154-91002.
Authors’ contributions statement
Ali Akbar Heidari Zefreh contributed in designing the experiment, practical process of microspore culture, practical process of flow cytometery, cytogenetic analysis, plant adaptation, greenhouse management, data analysis, and writing of the manuscript. Mehran E. Shariatpanahi (corresponding author) contributed in supervising the whole practical process of microspore culture, data analysis, and writing of the manuscript. Amir Mousavi contributed in the data analysis and editing of the manuscript. Sepideh Kalatejari contributed in providing the plant material seeds and materials needed for cytogenetic analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Néstor Carrillo
Rights and permissions
About this article
Cite this article
Heidari-Zefreh, A.A., Shariatpanahi, M.E., Mousavi, A. et al. Enhancement of microspore embryogenesis induction and plantlet regeneration of sweet pepper (Capsicum annuum L.) using putrescine and ascorbic acid. Protoplasma 256, 13–24 (2019). https://doi.org/10.1007/s00709-018-1268-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-018-1268-3