Abstract
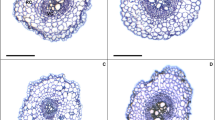
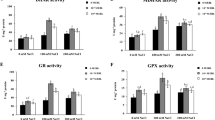
Brassinosteroids (BRs) can effectively alleviate the oxidative stress caused by Ca(NO3)2 in cucumber seedlings. The root system is an essential organ in plants due to its roles in physical anchorage, water and nutrient uptake, and metabolite synthesis and storage. In this study, 24-epibrassinolide (EBL) was applied to the cucumber seedling roots under Ca(NO3)2 stress, and the resulting chemical and anatomical changes were characterized to investigate the roles of BRs in alleviating salinity stress. Ca(NO3)2 alone significantly induced changes in the components of cell wall, anatomical structure, and expression profiles of several lignin biosynthetic genes. Salt stress damaged several metabolic pathways, leading to cell wall reassemble. However, EBL promoted cell expansion and maintained optimum length of root system, alleviating the oxidative stress caused by Ca(NO3)2. The continuous transduction of EBL signal thickened the secondary cell wall of casparian band cells, thus resisting against ion toxicity and maintaining water transport.







Similar content being viewed by others
Abbreviations
- BRs:
-
Brassinosteroids
- EBL:
-
24-Epibrassinolide
- BRI1:
-
Brassonosteroid insensitive 1
- BES1/BZR1:
-
bri1 EMS suppressor 1/brassinazole resistant 1
- PAL:
-
Phenylalanine ammonialyase
- F5H:
-
Ferulate-5-hydroxylas
- COMT:
-
Caffeicacid-3-O-methyltransferase
- CCoAOMT:
-
Caffeoyl-CoA-3-O-methyltransferase
- LAC:
-
Laccase
- LFA POD:
-
Lignin-forming anionic peroxidase
- BAK1:
-
BRI1-associated kinase 1
- BSU1:
-
bri1 suppressor 1
- BIN2:
-
Brassinosteroid insensitive 2
References
Agoda-Tandjawa G, Durand S, Gaillard C, Garnier C, Doublier JL (2012) Properties of cellulose/pectins composites: implication for structural and mechanical properties of cell wall. Carbohydr Polym 90(2):1081–1091. https://doi.org/10.1016/j.carbpol.2012.06.047
Bell AN, Magill E, Hallsworth JE, Timson DJ (2013) Effects of alcohols and compatible solutes on the activity of β-galactosidase. Appl Biochem Biotechnol 169(3):786–794. https://doi.org/10.1007/s12010-012-0003-3
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54(2):484–489. https://doi.org/10.1016/0003-2697(73)90377-1
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49(1):427–451. https://doi.org/10.1146/annurev.arplant.49.1.427
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6(11):850–861. https://doi.org/10.1038/nrm1746
Cosgrove DJ, Jarvis MC (2012) Comparative structure and biomechanics of plant primary and secondary cell walls. Front Plant Sci 3:204. https://doi.org/10.3389/fpls.2012.00204
de Lima RB, dos Santos TB, Vieira LG, de Lourdes Lúcio Ferrarese M, Ferrarese-Filho O, Donatti L, Boeger MR, de Oliveira Petkowicz CL (2014) Salt stress alters the cell wall polysaccharides and anatomy of coffee (Coffea arabica L.) leaf cells. Carbohydr Polym 112:686–694. https://doi.org/10.1016/j.carbpol.2014.06.042
Doblin MS, Pettolino F, Bacic A (2010) Plant cell walls: the skeleton of the plant world. Funct Plant Biol 37(5):357–381. https://doi.org/10.1071/FP09279
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Dwevedi A, Kayastha AM (2009) A beta-galactosidase from pea seeds (PsBGAL): purification, stabilization, catalytic energetics, conformational heterogeneity, and its significance. J Agric Food Chem 57(15):7086–7096. https://doi.org/10.1021/jf900874p
Friedrichsen DM, Joazeiro CA, Li J, Hunter T, Chory J (2000) Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase 1. Plant Physiol 123(4):1247–1255. https://doi.org/10.1104/pp.123.4.1247
Gibbs DJ, Coates JC (2014) AtMYB93 is an endodermis-specific transcriptional regulator of lateral root development in Arabidopsis. Plant Signal Behav 9(10):e970406. https://doi.org/10.4161/15592316.2014.970406
Hacham Y, Holland N, Butterfield C, Ubeda-Tomas S, Bennett MJ, Chory J, Savaldi-Goldstein S (2011) Brassinosteroid perception in the epidermis controls root meristem size. Development 138(5):839–848. https://doi.org/10.1242/dev.061804
Hossain Z, Mcgarvey B, Amyot L, Gruber M, Jung J, Hannoufa A (2012) DIMINUTO 1 affects the lignin profile and secondary cell wall formation in Arabidopsis. Planta 235(3):485–498. https://doi.org/10.1007/s00425-011-1519-4
Iiyama K, Wallis AFA (1988) An improved acetyl bromide procedure for determining lignin in woods and wood pulps. Wood Sci Technol 22(3):271–280. https://doi.org/10.1007/BF00386022
Iiyama K, Wallis AFA (1990) Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J Sci Food Agric 51(2):145–161. https://doi.org/10.1002/jsfa.2740510202
Jia XL, Wang GL, Xiong F, XR Y, ZS X, Wang F, Xiong AS (2015) De novo assembly, transcriptome characterization, lignin accumulation, and anatomic characteristics: novel insights into lignin biosynthesis during celery leaf development. Sci Rep 5(1):8259. https://doi.org/10.1038/srep08259
Jin H, Do J, Shin SJ, Choi JW, Choi YI, Kim W, Kwon M (2014) Exogenously applied 24-epi brassinolide reduces lignification and alters cell wall carbohydrate biosynthesis in the secondary xylem of Liriodendron tulipifera. Phytochemistry 101:40–51. https://doi.org/10.1016/j.phytochem.2014.02.003
Karahara I, Ikeda A, Kondo T, Uetake Y (2004) Development of the Casparian strip in primary roots of maize under salt stress. Planta 219(1):41–47. https://doi.org/10.1007/s00425-004-1208-7
Khan M, Rozhon W, Bigeard J, Pflieger D, Husar S, Pitzschke A, Teige M, Jonak C, Hirt H, Poppenberger B (2013) Brassinosteroid-regulated GSK3/shaggy-like kinases phosphorylate mitogen-activated protein (MAP) kinase kinases, which control stomata development in Arabidopsis thaliana. J Biol Chem 288(11):7519–7527. https://doi.org/10.1074/jbc.M112.384453
Kim HJ, Triplett B (2008) Involvement of extracellular Cu/Zn superoxide dismutase in cotton fiber primary and secondary cell wall biosynthesis. Plant Signal Behav 3(12):1119–1121. https://doi.org/10.4161/psb.3.12.7039
Kim TW, Guan S, Sun Y, Deng Z, Tang W, Shang JX, Sun Y, Burlingame AL, Wang ZY (2009) Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat Cell Biol 11(10):1254–1260. https://doi.org/10.1038/ncb1970
Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yoshida S, Chua NH (1998) The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell 10(10):1677–1690. https://doi.org/10.1105/tpc.10.10.1677
Lapierre C, Pollet B, Petit-Conil M, Toval G, Romero J, Pilate G, Leple JC, Boerjan W, Ferret VV, De Nadai V, Jouanin L (1999) Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid O-methyltransferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiol 119(1):153–163. https://doi.org/10.1104/pp.119.1.153
Li J, Chory J (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90(5):929–938. https://doi.org/10.1016/S0092-8674(00)80357-8
Lindner H, Müller LM, Boisson-Dernier A, Grossniklaus U (2012) CrRLK1L receptor-like kinases: not just another brick in the wall. Curr Opin Plant Biol 15(6):659–669. https://doi.org/10.1016/j.pbi.2012.07.003
Marshall CJ (1994) MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev 4(1):82–89. https://doi.org/10.1016/0959-437X(94)90095-7
Peterson CA, Steudle E (1993) Lateral hydraulic conductivity of early metaxylem vessels in Zea mays L. roots. Planta 189:288–297
Robbins NE, Trontin C, Duan L, Dinneny JR (2014) Beyond the barrier: communication in the root through the endodermis. Plant Physiol 166(2):551–559. https://doi.org/10.1104/pp.114.244871
Sánchez-Aguayo I, Rodríguez-Galán JM, García R, Torreblanca J, Pardo JM (2004) Salt stress enhances xylem development and expression of S-adenosyl-l-methionine synthase in lignifying tissues of tomato plants. Planta 220(2):278–285. https://doi.org/10.1007/s00425-004-1350-2
Savaldi-Goldstein S, Peto C, Chory J (2007) The epidermis both drives and restricts plant shoot growth. Nature 446(7132):199–202. https://doi.org/10.1038/nature05618
Schrick K, Fujioka S, Takatsuto S, Stierhof YD, Stransky H, Yoshida S, Jürgens G (2004) A link between sterol biosynthesis, the cell wall, and cellulose in Arabidopsis. Plant J 38(2):227–243. https://doi.org/10.1111/j.1365-313X.2004.02039.x
Schumacher K, Chory J (2000) Brassinosteroid signal transduction: still casting the actors. Curr Opin Plant Biol 3(1):79–84. https://doi.org/10.1016/S1369-5266(99)00038-2
Srivastava S, Vishwakarma RK, Arafat YA, Gupta SK, Khan BM (2015) Abiotic stress induces change in Cinnamoyl CoA reductase (CCR) protein abundance and lignin deposition in developing seedlings of Leucaena leucocephala. Physiol Mol Biol Plants 21(2):197–205. https://doi.org/10.1007/s12298-015-0289-z
Tsai CJ, Popko JL, Mielke MR, WJ H, Podila GK, Chiang VL (1998) Suppression of O-methyltransferase gene by homologous sense transgene in quaking aspen causes red-brown wood phenotypes. Plant Physiol 117(1):101–112. https://doi.org/10.1104/pp.117.1.101
You TT, Mao JZ, Yuan TQ, Wen JL, Xu F (2013) Structural elucidation of the lignins from stems and foliage of Arundo donax linn. J Agric Food Chem 61(22):5361–5370. https://doi.org/10.1021/jf401277v
Zhang GW, Liu ZL, Zhou JG, Zhu YL (2008) Effects of Ca(NO3)2 stress on oxidative damage, antioxidant enzymes activities and polyamine contents in roots of grafted and non-grafted tomato plants. Plant Growth Regul 56(1):7–19. https://doi.org/10.1007/s10725-008-9281-8
Zhao Q, Dixon RA (2011) Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci 16(4):227–233. https://doi.org/10.1016/j.tplants.2010.12.005
Zhong R, Ye Z (2007) Regulation of cell wall biosynthesis. Curr Opin Plant Biol 10(6):564–572. https://doi.org/10.1016/j.pbi.2007.09.001
Zhu XF, Lei GJ, Jiang T, Liu Y, Li GX, Zheng SJ (2012) Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 236(4):989–997. https://doi.org/10.1007/s00425-012-1652-8
Funding
This work was supported by the National Natural Science Foundation of China (No. 31471869, No. 31401919 and No. 31272209), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PDPA), China Agriculture Research System (CARS-25-C-03), and sponsored by the Research Fund for the Doctoral Program of Higher Education (20130097120015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Bhumi Nath Tripathi
Electronic supplementary material
Supplementary Table 1
(XLS 38 kb)
Rights and permissions
About this article
Cite this article
An, YH., Zhou, H., Yuan, YH. et al. 24-Epibrassinolide-induced alterations in the root cell walls of Cucumis sativus L. under Ca(NO3)2 stress. Protoplasma 255, 841–850 (2018). https://doi.org/10.1007/s00709-017-1187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1187-8