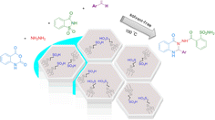
Abstract
Sulfenamide and its derivatives (S–N bond) have been synthesized with classical method in the literature. However, microwave-assisted synthesis of a series of N-(substituted phenylthio), N-(benzylthio), N-(cyclothio), and N-(2-mercaptobenzimidazolyl)amines has been not in the literature yet. They have been obtained from treating some amines (4 mmol) with thiophthalimides (PhthSR, 1 mmol) using sulfur transfer reagent in the presence of 2-ethoxyethanol (β-ee, neat) under microwave irradiation at 50 °C. The scope of this reaction was shown by the efficient synthesis of sulfenamides in good to excellent yields of 70–98% under free catalyst and ligand. Nine of the synthesized sulfenamide derivatives are novel. All of the thiols react with morpholine to give corresponding sulfenamides in excellent yields of 78–98%. Thiophenol, 4-methylthiophenol, 4-chlorothiophenol, and 4-fluorothiophenol react with cyclohexylamine to give corresponding sulfenamides in high yields 81–92%. Thiophenol, 4-methylthiophenol, 4-chlorothiophenol react with pyrrolidine to give corresponding sulfenamides in good yields of 70–76%. We observed that the reaction of t-butylamine with N-(phenylthio)phthalimide gave desired sulfenamide under microwave irradiation in the presence of DPPH as radical scavenger reagent in high yield of 93%. Aniline, benzylamine, 1-hexylamine, ethanolamine, diethylamine, N-ethyl-n-butylamine, N-ethylaniline, N-benzylmethylamine, t-butylamine react with thiols to give symmetrical disulfides instead of desired products under microwave irradiation, 2-ethoxyethanol as a solvent (neat), and at 50 °C. In this study, microwave-assisted synthesis method was compared with the classical method. All the products obtained were purified with chromatographic method and the analysis of these products was confirmed with IR, 1H NMR, 13C NMR spectroscopy, MS spectrometry, and elemental methods.
Graphical abstract

Similar content being viewed by others
References
Davis FA (1973) Int J Sulfur Chem 8:71
Davis FA (2006) J Org Chem 71:8993
Brito I, Restovic A, Pedreros S, Mancilla A, Vargas D, Leon Y, Ramirez E, Arias M, Brown K, Alvarez A, Arancibia A, Lopez-Rodriguez M (2003) J Chilean Chem Soc 48:51
Nti-Addae KW (2008) Synthesis and physicochemical characterization of sulfenamide prodrugs of antimicrobial oxazolidinones. PhD dissertation, Pharmaceutical Chemistry Department, University of Kansas
Koval IV (1996) Russ Chem Rev 65:421
Koval IV (1995) Russ Chem Rev 64:731
Koval IV (1990) Russ Chem Rev 59:396
Heimer NE, Field L (1970) J Org Chem 35:3012
Davis FA, Fretz ER, Horner CJ (1973) J Org Chem 38:690
Davis FA, Friedman AJ, Kluger EW, Skibo EB, Fretz ER, Milicia AP, Lemasters WC (1977) J Org Chem 42:967
Benati L, Montevecchi PC, Spagnolo P (1986) Tetrahedron Lett 27:1739
Kuehle E (1973) The chemistry of the sulfenic acids. Georg Thieme, Stuttgart
Craine L, Raban M (1989) J Am Chem Soc 89:689
Huttunen KM, Leppänen J, Laine K, Vepsäläinen J, Rautio J (2013) Eur J Pharm Sci 49:624
Caddick S (1995) Tetrahedron 51:10403
Valizadeh H, Dinparast L (2012) Monatsh Chem 143:251
Karakuş H, Dürüst Y (2017) Mol Divers 21:53
Adam D (2003) Nature 421:571
Küçükbay H, Yilmaz Ü, Yavuz K, Buğday N (2015) Turk J Chem 39:1265
Loupy A, Petit A, Hamelin F, Texier-Boullet F, Jacquault P, Mathe’ D (1998) Synthesis 1998:1213
Lidström P, Tierney J, Wathey B, Westman J (2001) Tetrahedron 57:9225
Varma RS (1999) Green Chem 1:43
Kappe CO (2004) Angew Chem Int Ed 43:6250
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, Oxford, p 2
Harpp DN, Back TG (1971) Tetrahedron Lett 52:4953
Kharasch K, Potempa SJ, Wehrmeister HL (1946) Chem Rev 39:269
Shimizu M, Fukazawa H, Shimada S, Abe Y (2006) Tetrahedron 62:2175
Senning A, Boureghda A, Abdel-Megeed MF, Jensen B, Nielsen B, Jensen AK (1991) Sulfur Lett 13:187
Davis FA, Nadir UK (1979) Org Prep Proced Int 11:33
Taniguchi N (2010) Eur J Org Chem 2010:2670
Taniguchi N (2017) Tetrahedron 73:2030
Rattanangkool E, Krailat W, Vilaivan T, Phuwapraisirisan P, Sukwattanasinitt M, Wacharasindhu S (2014) Eur J Org Chem 2014:4795
Klose J, Reese CB, Song Q (1997) Tetrahedron 53:14411
Kutuk H, Turkoz N (2011) Phosphorus Sulfur Silicon Relat Elem 186:1515
Kutuk H, Yakan H (2011) Phosphorus Sulfur Silicon Relat Elem 186:1460
Turkoz-Karakullukcu N, Yakan H, Ozturk S, Kutuk H (2013) Phosphorus Sulfur Silicon Relat Elem 188:1576
Taniguchi N (2007) Synlett 2007:1917
Dunbar JE, Rogers JH (1966) J Org Chem 31:2842
Kadoma Y, Murakami Y, Ogiwara T, Machino M, Yokoe I, Fujisawa S (2010) Molecules 15:1103
Fujisawa S, Kadoma Y, Yokoe I (2004) Chem Phys Lipids 130:189
Halliwell B (1994) Nutr Rev 52:257
Bondet V, Brand-Williams W, Berset C (1997) Lebensm Wiss Technol 30:609
Suwa S, Sakamoto T, Kikugawa Y (1999) Chem Pharm Bull 47:980
Tanaka T, Azuma T, Fang X, Uchida S, Iwata C, Ishida T, In Y, Maezaki N (2000) Synlett 2000:33
Clennan EL, Zhang H (1995) J Am Chem Soc 117:4218
Levchenko ES, Dubinina TN, Sereda SV, Antipin MY, Struchkov YT, Boldeskul IE (1987) Russ J Org Chem 23:86
Torii S, Tanaka H, Ukida X (1979) J Org Chem 44:1554
Maeda S, Samukawa S, Kobayashi H (1964) Benzimidazole derivatives. Patent JP 39015834, Aug 5, 1964; (1965) Chem Abstr 62:36857
Acknowledgements
We would like to thank Ondokuz Mayis University (Grant No. PYO.FEN.1904.10.024) for its financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yakan, H., Kütük, H. Comparison of conventional and microwave-assisted synthesis of some new sulfenamides under free catalyst and ligand. Monatsh Chem 149, 2047–2057 (2018). https://doi.org/10.1007/s00706-018-2261-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2261-4