Abstract
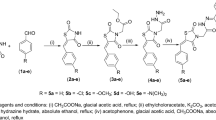
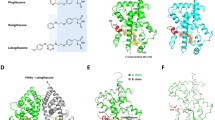
The present work describes the design, synthesis, molecular docking, biological evaluation, and assessment of structure–activity relationship of new derivatives based upon the molecular skeleton of the drug pioglitazone, a compound which is currently used for the management of type 2 diabetes mellitus. Pioglitazone has several side effects such as weight gain, edema, congestive heart failure, and bladder cancer. Therefore, there is a strong demand for identification of new lead candidates in the treatment of type 2 diabetes mellitus. A series of 24 compounds were prepared and evaluated for their peroxisome proliferator-activated receptor-γ (PPARγ) binding affinity assay and the IC50 values were determined. Among these compounds, six compounds exhibited promising IC50 values as compared to standard drugs pioglitazone and rosiglitazone. Furthermore, in order to confirm the target of these molecules, molecular docking study was carried out with peroxisome proliferator-activated receptor-γ (PPARγ) protein. Molecular modeling studies suggested that these compounds appropriately interact in the active sites of receptor.
Graphical abstract







Similar content being viewed by others
References
Espinosa JJR, Rios MY, Martínez SL, Vallejo FL, Medina-Franco JL, Paoli P, Camici G, Navarrete-Vázquez G, Ortiz-Andrade R, Estrada-Soto S (2011) Eur J Med Chem 46:2243
De-Fronzo RA (1992) Diabetologia 35:389.h
Reddy TN, Ravinder M, Bagul P, Ravikanti K, Bagul C, Nanubolu JB, Srinivas K, Banerjee SK, Rao VJ (2014) Eur J Med Chem 71:53
Gurram RM, Chakrabarti R, Reeba KV (2002) Bioorg Med Chem 10:2671
Cantello BCC, Cawthome MA, Haigh D, Hindley RM, Smith SA, Thurlby PL (1994) Bioorg Med Chem Lett 10:1181
Zimmet P, Alberti KGMM, Shaw J (2001) Nature 414:782
Ibrahim MK, Eissa IH, Abdallah AE, Metwaly AM, Radwan MM, ElSohly MA (2017) Bioorg Med Chem 25:1496
Turner NC (1996) Drug Discovery Today 1:109
Silink M, Tuomilehto J, Mbanya JC, Venkat Narayani KM, Fradkin J, Roglic G (2010) A prioritized research agenda for prevention and control of noncommunicable diseases. World Health Organization, Geneva
Shaw JE, Sicree RA, Zimmet PZ (2010) Diabetes Res Clin Pract 87:4
Camer D, Yu Y, Szabo A, Huang X (2014) Mol Nutr Food Res 58:1750
Harrichund P, Naicker S, Raal FJ (2008) J Endocrinol 13:3
Tokito A, Koriyama N, Ijuin A, Ogiso K, Nishio Y, Jougasaki M (2015) J Diabetes Mellitus 5:258
Kim H, Gim H, Yang M, Ryu J, Jeon R (2007) Heterocycles 71:2131
Fujita T, Sugiyama Y, Taketomi S, Sohda T, Kawamatsu T, Iwatsuka H, Suzuoki Z (1983) Diabetes 32:804
Spiegelman BM (1998) Diabetes 47:507
Bogacka I, Xie H, Bray GA, Smith SR (2004) Diabetes Care 27:1660
Vidal-Puig AJ, Considine RV, Jimenez-Liñan M, Ariel Werman A, Pories WJ, Caro JF, Flier JS (1997) J Clin Invest 99:2416
Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Kliewer SA (1995) J Biol Chem 270:12953
Lehrke M, Lazar MA (2005) Cell 123:993
Elbrecht A, Chen Y, Cullinan CA, Hayes N, Leibowitz MD, Moller DE, Berger J (1996) Biochem Biophys Res Commun 224:431
Kersten S, Desvergne B, Wahli W (2000) Nature 405:421
Kaserer T, Obermoser V, Weninger A, Gust R, Schuster D (2016) Eur J Med Chem 124:49
Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F (2008) Nature 456:350
Usui S, Suzuki T, Hattori Y, Etoh K, Fujieda H, Nishizuka M, Imagawa M, Nakagawa H, Kohda K, Miyata N (2005) Bioorg Med Chem Lett 15:1547
Grillier-Vuissoz I, Mazerbourg S, Boisbrun M, Kuntz S, Chapleur Y, Flament S (2012) J Carcinog Mutagen S8:002
Uchiyama M, Koda H, Fischer T, Mueller J, Yamamura N, Oguchi M, Iwabuchi H, Okazaki O, Izumi T (2011) Drug Metab Dispos 39:1311
Patel KP, Joshi HM, Majmudar FD, Patel VJ (2013) J Med Sci 2:6
Pastromas S, Kouloris S (2006) Hellenic J Cardiol 47:352
Hauner H (2002) Diabetes Metab Res Rev 18:S10
Martens FMAC, Visseren FLJ, Lemay J, de Koning EJP, Rabelink TJ (2002) Drugs 62:1463
Lebovitz HE, Banerji MA (2001) Recent Prog Horm Res 56:265
Komers R, Vrana A (1998) Physiol Res 47:215
Ye J (2011) Acta Pharm Sin B 1:137
Madivada LR, Anumala RR, Gilla G, Alla S, Charagondla K, Kagga M, Bhattacharya A, Bandichhor R (2009) Org Process Res Dev 13:1190
Al-Rashood KA, Abdel-Aziz HA (2010) Molecules 15:3775
Turek M, Szczęsna D, Koprowski M, Bałczewski P (2017) Beilstein J Org Chem 13:451
Prashantha Kumar BR, Soni M, Santhosh Kumar S, Singh K, Patil M, Nasir Baig RB, Adhikary L (2011) Eur J Med Chem 6:835
Darwish KM, Salama I, Mostafa SM, Gomaa MS, Helal MA (2016) Eur J Med 109:157
Hu Q, Yin L, Hartmann RW (2012) J Med Chem 55:7080
Oshiro Y, Sato S, Kurahashi N, Tanaka T, Kikuchi T, Tottori K, Uwahodo Y, Nishi T (1998) J Med Chem 41:658
Wu Y, Tai HH, Cho H (2010) Bioorg Med Chem 18:1428
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) J Comput Chem 30:2785
Huang B, Schroeder M (2006) BMC Struct Biol 6:19
Trott O, Olson AJ (2010) J Comput Chem 31:455
Acknowledgements
One of the authors, RNC is thankful to Mr. Awatar singh and Manish kumar, SAIF, Punjab University, Chandigarh, for providing spectral data of all the synthesized compounds. The author is also greatly thankful to management of Ind-Swift laboratory Ltd., for providing all necessary facility to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaturvedi, R.N., Pendem, K., Patel, V.P. et al. Design, synthesis, molecular docking, and in vitro antidiabetic activity of novel PPARγ agonist. Monatsh Chem 149, 2069–2084 (2018). https://doi.org/10.1007/s00706-018-2207-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2207-x