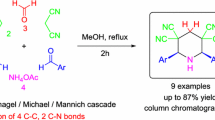
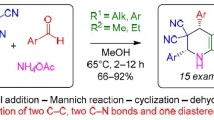
Abstract
An effective and facile multicomponent method for the synthesis of polysubstituted piperidines is described. The Michael–Mannich type cascade of benzylidenemalononitriles with aromatic aldehydes and ammonium acetate or aqueous ammonia provides convenient access to the stereoselective synthesis of 3,3,5,5-tetracyano-2,4,6-triarylpiperidines in good to excellent yields in one-pot manner. Ammonium acetate or aqueous ammonia plays a role both as a catalyst and as a nitrogen source. It is established that the reaction proceeds via sequence of equilibriums and a competitive mechanisms are implemented.
Graphical abstract




Similar content being viewed by others
References
Zhu J, Wang Q, Wang M (2015) Multicomponent reactions in organic synthesis. Wiley-VCH, Weinheim
Graaff C, Ruijter E, Orru RVA (2012) Chem Soc Rev 41:3969
Marson CM (2012) Chem Soc Rev 41:7712
Rotstein BH, Zaretsky S, Rai V, Yudin AK (2014) Chem Rev 114:8323
Dömling A (2015) Chem Rec 15:981
Elinson MN, Vereshchagin AN, Ryzhkov FV (2016) Chem Rec 16:1950
Gamez-Montano R, Zhu J (2002) Chem Commun 20:2248
Touré BB, Hall DG (2009) Chem Rev 109:4439
Ruijter E, Orru RVA (2013) Drug Discov Today 10:e15
Zarganes T, Dömling A (2014) Org Chem Front 1:834
Ganem B (2009) Acc Chem Res 42:463
Ruijter E, Scheffelaar R, Orru RVA (2011) Angew Chem Int Ed 50:6234
Shiri M (2012) Chem Rev 112:3508
Brauch S, van Berkel SS, Westermann B (2013) Chem Soc Rev 42:4948
Allais C, Grassot JM, Rodriguez J, Constantieux T (2014) Chem Rev 114:10829
Vereshchagin AN (2017) Russ Chem Bull 66:1765
Weber L (2002) Curr Med Chem 9:2085
Weber L (2002) Drug Discov Today 7:143
Slobbe P, Ruijter E, Orru RVA (2012) MedChemCommun 3:1189
Dömling A, Wang W, Wang K (2012) Chem Rev 112:3083
Koszytkowska-Stawińska M, Buchowicz W (2014) Beilstein J Org Chem 10:1706
Dömling A (2006) Chem Rev 106:17
Levi L, Müller TJJ (2016) Chem Soc Rev 45:2825
Bienayme H, Bouzid K (1998) Angew Chem Int Ed 37:2234
Ugi I, Heck S (2001) Comb Chem High Throughput Screen 4:1
Gerencsér J, Dormán G, Darvas F (2006) QSAR Comb Sci 25:439
Martin KS, Di Maso MJ, Fettinger JC, Shaw JT (2013) ACS Comb Sci 15:356
Rani GU, Kumar SV, Bharkavi C, Menéndez JC, Perumal S (2016) ACS Comb Sci 18:337
Elbein DA, Molyneux R (1987) In: Palletier SW (ed) Alkaloids: chemical and biological perspectives, vol 57. Wiley, New York
Pinder AR (1992) Nat Prod Rep 9:17
Felpin FX, Lebreton J (2004) Tetrahedron 60:10127
Ho B, Crider AM, Stables JP (2001) Eur J Med Chem 36:265
Maier CA, Wunsch B (2002) J Med Chem 45:438
Weis R, Schweiger K, Faist J, Rajkovic E, Kungl AJ, Fabian WMF, Schunack W, Seebacher W (2008) Bioorg Med Chem 16:10326
Misra M, Pandey SK, Pandey VP, Pandey J, Tripathi R, Tripathi RP (2009) Bioorg Med Chem 17:625
Wang W, Hu Y (2012) Med Res Rev 32:1159
Tanaka R, Rubio A, Harn NK, Gernert D, Grese TA, Eishima J, Hara M, Yoda N, Ohashi R, Kuwabara T, Soga S, Akinaga S, Nara S, Kanda Y (2007) Bioorg Med Chem 5:1363
Reshetov PV, Kriven’ko AP, Boreko EI, Vladyko GV, Korobchenko LV (1990) Pharm Chem J 24:889
Kudou T, Tanima D, Masuzawa Y, Yano T (2011) Ortho-substituted haloalkylsulfonanilide derivatives as herbicides. Eur Patent 2336104, 22 June 2011; (2010) Chem Abstr 152:357881
Guareschi L, Grande E (1898) Chem Zentralbl 69:544
Robinson R (1917) J Chem Soc 111:762
Schöpf C (1937) Angew Chem 50:779
Zhu W, Mena M, Jnoff E, Sun N, Pasau P, Ghosez L (2009) Angew Chem Int Ed 48:5880
Zhou Z, Liu H, Sun Q, Li Y, Yang J, Liu J, Yan P, Wang C (2005) Synlett 23:2255
Liu H, Zhou Z, Sun Q, Li Y, Li Y, Liu J, Yan P, Wang D, Wang C (2012) ACS Comb Sci 14:366
Li Y, Sun Q, Zhou Z, Liu J, Yang J, Wang C (2013) Monatsh Chem 144:1031
Li Y, Xue Z, Ye W, Liu J, Yao J, Wang C (2014) ACS Comb Sci 16:113
Elinson MN, Feducovich SK, Vereshchagin AN, Dorofeev AS, Dmitriev DE, Nikishin GI (2003) Russ Chem Bull Int Ed 52:2235
Elinson MN, Feducovich SK, Zaimovskaya TA, Vereshchagin AN, Gorbunov SV, Nikishin GI (2005) Russ Chem Bull Int Ed 54:1593
Vereshchagin AN, Elinson MN, Stepanov NO, Nikishin GI (2009) Mendeleev Commun 19:324
Elinson MN, Vereshchagin AN, Stepanov NO, Ilovaisky AI, Vorontsov AY, Nikishin GI (2009) Tetrahedron 65:6057
Elinson MN, Vereshchagin AN, Feducovich SK, Zaimovskaya TA, Starikova ZA, Belyakov PA, Nikishin GI (2007) Tetrahedron Lett 48:6614
Elinson MN, Feducovich SK, Zaimovskaya TA, Vereshchagin AN, Nikishin GI (2003) Russ Chem Bull Int Ed 52:2241
Vereshchagin AN, Elinson MN, Zaimovskaya TA, Nikishin GI (2008) Tetrahedron 64:9766
Vereshchagin AN, Elinson MN, Dorofeeva EO, Stepanov NO, Zaimovskaya TA, Nikishin GI (2013) Tetrahedron 69:1945
Elinson MN, Dorofeeva EO, Vereshchagin AN, Korshunov AD, Egorov MP (2016) Res Chem Intermed 42:2191
Lemek T, Mayr H (2003) J Org Chem 68:6880
Takajo T, Kambe S (1980) Synthesis 1980:833
Chakrabarty M, Karmakar S, Arima S, Harigaya Y (2007) Heterocycles 73:795
Geissler G, Hippius M, Tomaschewski G (1982) J Prakt Chem 324:910
Rajasekaran K, Sarathi A, Ramalakshmi S (2008) J Chem Sci 120:475
Elinson MN, Feducovich SK, Zaimovskaya TA, Vereshchagin AN, Nikishin GI (2005) Russ Chem Bull Int Ed 54:673
Vereshchagin AN, Elinson MN, Egorov MP (2015) RSC Adv 5:98522
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann HJ (2009) Appl Cryst 42:339
Sheldrick GM (2008) Acta Crystallogr A64:112
Acknowledgements
This work was supported by the Russian Science Foundation (RSF Grant 17-73-20260).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vereshchagin, A.N., Karpenko, K.A., Elinson, M.N. et al. Stereoselective one-pot synthesis of polycyanosubstituted piperidines. Monatsh Chem 149, 1979–1989 (2018). https://doi.org/10.1007/s00706-018-2187-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2187-x