Abstract
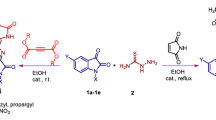
The new anthraquinonylhydrazones were obtained by the interaction of 9,10-dioxoanthracene-1-diazonium sulfates with a number of α- and β-carbonyl-containing compounds under modified conditions of the Japp–Klingemann reaction, and a probable mechanism of the formation has been proposed. It was found that hydrazones, unsaturated in the second position of the anthraquinone ring, containing acetyl or ethoxycarbonyl moieties in the ylidene part of the molecule, are capable of eliminating these fragments. It has been experimentally established that hydrazones, free rotation around the N=C bond of which is possible, exist as one isomer due to the presence of an intramolecular hydrogen bond in the molecule. The anthraquinonylhydrazone of dimedone with action against the bacteria strains of Staphylococcus aureus 209-P, Mycobacterium luteum B-917, and fungus Candida tenuis VKM Y-70 was found. The hydrazones of dimedone and barbituric acid with a higher trolox equivalent antioxidant coefficients of antioxidant action were found using CUPRAC assay. In addition, the hydrazones of dimedone and barbituric acid exhibited better activity against catalase enzyme. Correlations between the structure of the synthesized hydrazones and their antioxidant activity have been defined.
Graphical abstract




Similar content being viewed by others
References
Belskaya N, Dehaen W, Bakulev V (2010) Arkivoc 2010:275
Ali R, Marella A, Alam T, Naz R, Akhter M, Shaquiquzzaman M, Saha R, Tanwar O, Alam M, Hooda J (2012) Indones J Pharm 23:193
Antonini I, Polucci P, Cola D, Palmieri G, Martelli S, Bontemps-Gracz M (1993) Farmaco 48:1641
Loskutov VA (2000) Russ J Org Chem 36:1478
Vaisburg AF, Etzlstorfer C, Falk H (1994) Monatsh Chem 125:1121
Bulhakova NA (2002) Synthesis, structure and properties of some 9,10-anthraquinone derivatives containing a nitrogen–nitrogen bond. Ph.D. thesis, Krasnoyarsk State Pedagogical University named after V.P. Astafieva, Krasnoyarsk ([in Russian])
Vorob’eva SL, Buyanov VN, Levina II, Suvorov NN (1989) Chem Heterocycl Compd 25:58
Kim MK, Wiemer DF (2004) Tetrahedron Lett 45:4977
Stasevych MV, Zvarych VI, Lunin VV, Vovk MV, Novikov VP (2017) Russ J Org Chem 53:468
Stasevych MV, Zvarych VI, Lunin VV, Khomyak SV, Vovk MV, Novikov VP (2017) Chem Heterocycl Compd 53:927
Zvarych V, Stasevych M, Lunin V, Deniz NG, Sayil C, Ozyurek M, Guclu K, Vovk M, Novikov V (2016) Monatsh Chem 147:2093
Butler RN, Quinn KF, Welke B (1992) J Chem Soc Chem Commun 1992:1481
Allen FH, Groom CR, Liebeschuetz JW, Bardwell DA, Olsson TSG, Wood JPA (2012) Chem Inf Model 52:857
Landge SM, Aprahamian I (2009) J Am Chem Soc 131:18269
Su X, Aprahamian I (2011) Org Lett 13:30
Landge SM, Tkatchouk E, Benítez D, Lanfranchi DA, Elhabiri M, Goddard WA, Aprahamian I (2011) J Am Chem Soc 133:9812
He L, Lu L, Zhang S, Freeman HS (2010) Color Technol 126:92
NCCLS (1990) Performance Standards for antimicrobial disk susceptibility tests, 4th edn: Approved standards. Document M2-A4, Villanova, PA
NCCLS (1998) Reference Method for broth dilution antifungal susceptibility testing of conidium forming filamentous fungi: proposed standard. Document M38-P, Wayne, PA
Apak R, Güçlü K, Özyürek M, Karademir SE (2004) J Agric Food Chem 52:7970
Bekdeşer B, Özyürek M, Güçlü K, Alkan FÜ, Apak R (2014) Spectrochim Acta Part A 132:485
Acknowledgements
The authors would like to express their gratitude to the Ministry of Education and Science of Ukraine, Scientific Research Project (Project number: 0116U004138) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stasevych, M., Zvarych, V., Lunin, V. et al. Synthesis and investigation of antimicrobial and antioxidant activity of anthraquinonylhydrazones. Monatsh Chem 149, 1111–1119 (2018). https://doi.org/10.1007/s00706-018-2157-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2157-3