Abstract
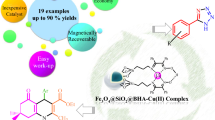
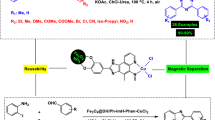
The Fe3O4@SiO2/Salen-Cu(II) nanocatalyst is reported as a thermally and air-stable, economical, and magnetically recoverable heterogeneous catalyst for the selective and efficient N-(hetero)arylation of imidazole. Only by adding a small amount of the catalyst (0.4 mol% Cu) to the reactants and heating under air, the new presented method provides a variety of functionalized and hindered N-(hetero)arylimidazoles in good to excellent yields within short reaction times. The catalyst could be easily recovered with the aid of a permanent magnet and reused up to five consecutive runs without significant loss of activity. Also, the leaching of Cu was negligible after the fifth recycle. Particularly, using either (hetero)aryl iodides or bromides as arylating agents and the need of only small amount of the magnetically recoverable heterogeneous copper-based nanocatalyst make this method low-cost, environmentally benign, and easy to use.
Graphical abstract



Similar content being viewed by others
References
Grauer A, Späth A, Ma D, König B (2009) Chem Asian J 4:1134
Güngör T, Fouquet A, Teulon JM, Provost D, Cazes M, Cloarec A (1992) J Med Chem 35:4455
Herrmann WA (2002) Angew Chem Int Ed 41:1290
Sircar I, Duell BL, Bobowski G, Bristol JA, Evans DB (1985) J Med Chem 28:1405
Lo YS, Nolan JC, Maren TH, Welstead WJ Jr, Gripshover DF, Shamblee DA (1992) J Med Chem 35:4790
Rani N, Sharma A, Singh R (2013) Mini Rev Med Chem 13:1812
Heeres J, Backx LJJ, Mostmans JH, Cutsem JV (1979) J Med Chem 22:1003
Al-Masoudi NA, Al-Soud YA, Kalogerakis A, Pannecouque C, De Clercq E (2006) Chem Biodivers 3:515
Leeson PD, Springthorpe B (2007) Nat Rev Drug Discov 6:881
Nyce GW, Glauser T, Connor EF, Möck A, Waymouth RM, Hedrick JL (2003) J Am Chem Soc 125:3046
Harkal S, Rataboul F, Zapf A, Fuhrmann C, Riermeier T, Monsees A, Beller M (2004) Adv Synth Catal 346:1742
Suárez-Pantiga S, Rubio E, Alvarez-Rúa C, González JM (2009) Org Lett 11:13
Venuti MC, Stephenson RA, Alvarez R, Bruno JJ, Strosberg AM (1988) J Med Chem 31:2136
Priyarega S, Raja DS, Babu SG, Karvembu R, Hashimoto T, Endo A, Natarajan K (2012) Polyhedron 34:143
Kiyomori A, Marcoux JF, Buchwald SL (1999) Tetrahedron Lett 40:2657
Wu CD, Li L, Shi LX (2009) Dalton Trans 2009:6790
López-Alvarado P, Avendaño C, Menéndez JC (1995) J Org Chem 60:5678
Kang SK, Lee SH, Lee D (2000) Synlett 2000:1022
Fedorov AY, Finet JP (1999) Tetrahedron Lett 40:2747
Farahat AA, Boykin DW (2014) Tetrahedron Lett 55:3049
Kantam ML, Yadav J, Laha S, Sreedhar B, Jha S (2007) Adv Synth Catal 349:1938
Yang Q, Wang Y, Yang L, Zhang M (2013) Tetrahedron 69:6230
Xu ZL, Li HX, Ren ZG, Du WY, Xu WC, Lang JP (2011) Tetrahedron 67:5282
Liu YS, Liu Y, Ma XW, Liu P, Xie JW, Dai B (2014) Chin Chem Lett 25:775
Polshettiwar V, Luque R, Fihri A, Zhu H, Bouhrara M, Basset JM (2011) Chem Rev 111:3036
Astruc D, Lu F, Aranzaes JR (2005) Angew Chem Int Ed 44:7852
Rout L, Jammi S, Punniyamurthy T (2007) Org Lett 9:3397
Safaei-Ghomi J, Zahedi S, Ghasemzadeh MA (2014) Monatsh Chem 145:1191
Mohaqeq M, Safaei-Ghomi J (2015) Monatsh Chem 146:1581
Alonso F, Riente P, Sirvent JA, Yus M (2010) Appl Catal A Gen 378:42
Rostamizadeh S, Abdollahi F, Shadjou N, Amani AM (2013) Monatsh Chem 144:1191
Gupta AK, Gupta M (2005) Biomaterials 26:3995
Harrison RJ, Dunin-Borkowski RE, Putnis A (2002) Proc Natl Acad Sci USA 99:16556
Wu W, He Q, Jiang C (2008) Nanoscale Res Lett 3:397
Esmaeilpour M, Sardarian AR (2014) Green Chem Lett Rev 7:301
Esmaeilpour M, Javidi J, Nowroozi-Dodeji F, Hassannezhad H (2014) J Iran Chem Soc 11:1703
Esmaeilpour M, Javidi J (2015) J Chin Chem Soc 62:614
Esmaeilpour M, Javidi J (2015) J Chin Chem Soc 62:328
Esmaeilpour M, Javidi J, Zahmatkesh S (2016) Appl Organomet Chem 30:897
Panda N, Jena AK, Mohapatra S, Rout SR (2011) Tetrahedron Lett 52:1924
Nador F, Volpe MA, Alonso F, Radivoy G (2014) Tetrahedron 70:6082
Sivakami R, Babu SG, Dhanuskodi S, Karvembu R (2015) RSC Adv 5:8571
Liu Y, Zhang Q, Ma X, Liu P, Xie J, Dai B, Liu Z (2013) Int J Org Chem 3:185
Dehghani F, Sardarian AR, Esmaeilpour M (2013) J Organomet Chem 743:87
Cristau HJ, Cellier PP, Spindler JF, Taillefer M (2004) Chem Eur J 10:5607
Emel’yanenko VN, Kaliner M, Strassner T, Verevkin SP (2017) Fluid Phase Equilib 433:40
Lv X, Wang Z, Bao W (2006) Tetrahedron 62:4756
Altman RA, Buchwald SL (2006) Org Lett 8:2779
Yang K, Qiu Y, Li Z, Wang Z, Jiang S (2011) J Org Chem 76:3151
Inamoto K, Nozawa K, Kadokawa J, Kondo Y (2012) Tetrahedron 68:7794
Zhang H, Cai Q, Ma D (2005) J Org Chem 70:5164
Xue F, Cai C, Sun H, Shen Q, Rui J (2008) Tetrahedron Lett 49:4386
Joseph PJA, Priyadarshini S, Kantam ML, Maheswaran H (2011) Catal Sci Technol 1:234
Hosseini-Sarvari M, Moeini F (2014) RSC Adv 4:7321
Esmaeilpour M, Sardarian AR, Jarrahpour A, Ebrahimi E, Javidi J (2016) RSC Adv 6:43376
Deng Y, Qi D, Deng C, Zhang X, Zhao D (2008) J Am Chem Soc 130:28
Acknowledgements
We would like to gratefully acknowledge financial support of this work by research council of Shiraz University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sardarian, A.R., Zohourian-Mashmoul, N. & Esmaeilpour, M. Salen complex of Cu(II) supported on superparamagnetic Fe3O4@SiO2 nanoparticles: an efficient and magnetically recoverable catalyst for N-arylation of imidazole with aryl halides. Monatsh Chem 149, 1101–1109 (2018). https://doi.org/10.1007/s00706-018-2148-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2148-4