Abstract
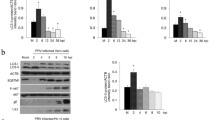
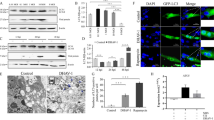
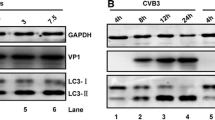
Autophagy is a homeostatic process that has been shown to be vital in the innate immune defense against pathogens. However, little is known about the regulatory role of autophagy in porcine teschovirus 2 (PTV-2) replication. In this study, we found that PTV-2 infection induces a strong increase in GFP-LC3 punctae and endogenous LC3 lipidation. However, PTV-2 infection did not enhance autophagic protein degradation. When cellular autophagy was pharmacologically inhibited by wortmannin or 3-methyladenine, PTV-2 replication increased. The increase in virus yield via autophagy inhibition was further confirmed by silencing atg5, which is required for autophagy. Furthermore, PTV-2 replication was suppressed when autophagy was activated by rapamycin. Together, the results suggest that PTV-2 infection activates incomplete autophagy and that autophagy then inhibits further PTV-2 replication.



Similar content being viewed by others
References
Knowles NJ, Hovi T, Hyypiä T (2012) Family Picornaviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (eds) Classification and nomenclature of viruses: ninth report of the international committee on taxonomy of viruses. Elsevier, San Diego, pp 855–880
Chiu SC, Hu SC, Chang CC, Chang CY, Huang CC, Pang VF, Wang FI (2012) The role of porcine teschovirus in causing diseases in endemically infected pigs. Vet Microbiol 161:88–95
Tsai ATH, Kuo CC, Kuo YC, Yang JL, Chang CY, Wang FI (2016) The urinary shedding of porcine teschovirus in endemic field situations. Vet Microbiol 182:150–155
Chiu SC, Yang CL, Chen YM, Hu SC, Chiu KC, Lin YC, Chang CY, Wang FI (2014) Multiple models of porcine teschovirus pathogenesis in endemically infected pigs. Vet Microbiol 168:69–77
Jones TH, Muehlhauser V (2017) F-coliphages, porcine adenovirus and porcine teschovirus as potential indicator viruses of fecal contamination for pork carcass processing. Int J Food Microbiol 241:237–243
Wang B, Tian Z-J, Gong D-Q, Li D-Y, Wang Y, Chen J-Z, An T-Q, Peng J-M, Tong G-Z (2010) Isolation of serotype 2 porcine teschovirus in China: evidence of natural recombination. Vet Microbiol 146:138–143
Jackson WT (2015) Viruses and the autophagy pathway. Virology 479:450–456
Orvedahl A, MacPherson S, Sumpter R, Tallóczy Z, Zou Z, Levine B (2010) Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe 7:115–127
Orvedahl A, Alexander D, Tallóczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B (2007) HSV-1 ICP34. 5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23–35
McKnight KL, Lemon SM (2017) Virology: Ins and outs of picornaviruses. Nature 541:299–300
Lai JK, Sam I, Chan YF (2016) The autophagic machinery in enterovirus infection. Viruses 8:32
Zhu B, Xu F, Li J, Shuai J, Li X, Fang W (2012) Porcine circovirus type 2 explores the autophagic machinery for replication in PK-15 cells. Virus Res 163:476–485
Hu B, Zhang Y, Jia L, Wu H, Fan C, Sun Y, Ye C, Liao M, Zhou J (2015) Binding of the pathogen receptor HSP90AA1 to avibirnavirus VP2 induces autophagy by inactivating the AKT-MTOR pathway. Autophagy 11:503–515
Levine B, Klionsky DJ (2017) Autophagy wins the 2016 Nobel Prize in Physiology or Medicine: Breakthroughs in baker’s yeast fuel advances in biomedical research. Proc Natl Acad Sci USA 114:201–205
Shibutani ST, Saitoh T, Nowag H, Münz C, Yoshimori T (2015) Autophagy and autophagy-related proteins in the immune system. Nat Immunol 16:1014
Kang Y, Yuan R, Xiang B, Zhao X, Gao P, Dai X, Liao M, Ren T (2017) Newcastle disease virus-induced autophagy mediates antiapoptotic signaling responses in vitro and in vivo. Oncotarget. https://doi.org/10.18632/oncotarget.18169
Cheng J-H, Sun Y-J, Zhang F-Q, Zhang X-R, Qiu X-S, Yu L-P, Wu Y-T, Ding C (2016) Newcastle disease virus NP and P proteins induce autophagy via the endoplasmic reticulum stress-related unfolded protein response. Sci Rep 6:24721
Sun Y, Yu S, Ding N, Meng C, Meng S, Zhang S, Zhan Y, Qiu X, Tan L, Chen H, Song C, Ding C (2014) Autophagy Benefits the Replication of Newcastle Disease Virus in Chicken Cells and Tissues. J Virol 88:525–537
Green AM, Beatty PR, Hadjilaou A, Harris E (2014) Innate immunity to dengue virus infection and subversion of antiviral responses. J Mol Biol 426:1148–1160
Jin R, Zhu W, Cao S, Chen R, Jin H, Liu Y, Wang S, Wang W, Xiao G (2013) Japanese encephalitis virus activates autophagy as a viral immune evasion strategy. PLoS One 8:e52909
Li J-K, Liang J-J, Liao C-L, Lin Y-L (2012) Autophagy is involved in the early step of Japanese encephalitis virus infection. Microbes Infect 14:159–168
Chan ST, Lee J, Narula M, Ou J-HJ (2016) Suppression of Host Innate Immune Response by Hepatitis C Virus via Induction of Autophagic Degradation of TRAF6. J Virol 90:10928–10935
Dreux M, Chisari F (2009) Autophagy proteins promote hepalitis C virus replication. Autophagy 5:1224–1225
Tallóczy Z, Virgin I, Herbert Levine B (2006) PKR-dependent xenophagic degradation of herpes simplex virus type 1. Autophagy 2:24–29
Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar S (2005) Autophagy regulates programmed cell death during the plant innate immune response. Cell 121:567–577
Mateo R, Nagamine CM, Spagnolo J, Méndez E, Rahe M, Gale M, Yuan J, Kirkegaard K (2013) Inhibition of cellular autophagy deranges dengue virion maturation. J Virol 87:1312–1321
Richards AL, Jackson WT (2013) Behind closed membranes: the secret lives of picornaviruses? PLoS Pathog 9:e1003262
Huang SC, Chang CL, Wang PS, Tsai Y, Liu HS (2009) Enterovirus 71-induced autophagy detected in vitro and in vivo promotes viral replication. J Med Virol 81:1241–1252
O’donnell V, Pacheco JM, LaRocco M, Burrage T, Jackson W, Rodriguez LL, Borca MV, Baxt B (2011) Foot-and-mouth disease virus utilizes an autophagic pathway during viral replication. Virology 410:142–150
Berryman S, Brooks E, Burman A, Hawes P, Roberts R, Netherton C, Monaghan P, Whelband M, Cottam E, Elazar Z (2012) Foot-and-mouth disease virus induces autophagosomes during cell entry via a class III phosphatidylinositol 3-kinase-independent pathway. J Virol 86:12940–12953
Zhang Y, Li Z, Ge X, Guo X, Yang H (2011) Autophagy promotes the replication of encephalomyocarditis virus in host cells. Autophagy 7:613–628
Bird SW, Maynard ND, Covert MW, Kirkegaard K (2014) Nonlytic viral spread enhanced by autophagy components. Proc Natl Acad Sci USA 111:13081–13086
Quiner CA, Jackson WT (2010) Fragmentation of the Golgi apparatus provides replication membranes for human rhinovirus 1A. Virology 407:185–195
Delorme-Axford E, Morosky S, Bomberger J, Stolz DB, Jackson WT, Coyne CB (2014) BPIFB3 regulates autophagy and coxsackievirus B replication through a noncanonical pathway independent of the core initiation machinery. MBio 5:e02114–e02147
Taylor MP, Kirkegaard K (2008) Potential subversion of autophagosomal pathway by picornaviruses. Autophagy 4:286–289
Münz C (2016) Autophagy beyond intracellular MHC class II antigen presentation. Trends Immunol 37:755–763
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun J-A, Outzen H, Øvervatn A, Bjørkøy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
O’Connell D, Liang C (2016) Autophagy interaction with herpes simplex virus type-1 infection. Autophagy 12:451–459
Gladue D, O’donnell V, Baker-Branstetter R, Holinka L, Pacheco J, Fernandez-Sainz I, Lu Z, Brocchi E, Baxt B, Piccone M (2012) Foot-and-mouth disease virus nonstructural protein 2C interacts with Beclin1, modulating virus replication. J Virol 86:12080–12090
Dong X, Levine B (2013) Autophagy and viruses: adversaries or allies? J Innate Immun 5:480–493
Jackson WT, Giddings TH Jr, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K (2005) Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol 3:e156
Cherry S, Kunte A, Wang H, Coyne C, Rawson RB, Perrimon N (2006) COPI activity coupled with fatty acid biosynthesis is required for viral replication. PLoS Pathog 2:e102
Levine B, Deretic V (2007) Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol 7:767–777
Nardacci R, Ciccosanti F, Marsella C, Ippolito G, Piacentini M, Fimia GM (2017) Role of autophagy in HIV infection and pathogenesis. J Intern Med 281:422–432
Shoji-Kawata S, Sumpter R Jr, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D (2013) Identification of a candidate therapeutic autophagy–inducing peptide. Nature 494:201
Acknowledgements
This work is partly supported by the important agriculture subject fund from Department of S&T of Zhejiang Province (2015C02044), Department of Education of Zhejiang Province (Y201635576), the Agricultural Technology Extension Funds of Zhejiang University, Dabei Agricultural Discipline Development and Talent Training Fund (2017ZDNT004), and three rural six party funds for Xiaoliang Li.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
All animal studies were approved by the Animal Care and Use Committee of Zhejiang University in accordance with the Chinese guidelines for the care and use of laboratory animals (Permit Number: 2016101098).
Additional information
Handling Editor Zhenhai Chen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gu, Y., Zhou, Y., Shi, X. et al. Porcine teschovirus 2 induces an incomplete autophagic response in PK-15 cells. Arch Virol 163, 623–632 (2018). https://doi.org/10.1007/s00705-017-3652-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3652-2