Abstract
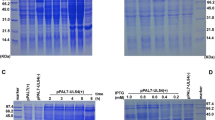
The pseudorabies virus (PRV) glycoprotein known as gG is generally regarded as an early protein, and the immediate early IE180 protein regulates its expression during infection. This study, however, provides evidence that although induction by IE180 is observed, the expression of a marker protein (EGFP), or gG itself, under the control of the gG promoter, can also occur independently of the expression of IE180. This result was demonstrated both with transient transfection assays using plasmids and with viral infections. In transient transfections, the expression under control of the gG promoter depends on the cell type and surprisingly, can be 1.3-fold higher than the expression under the control of the IE180 promoter in Hela Tet-Off cells. Recombinant PRV S3 was constructed by replacing gE in the PRV genome with a chimeric transgene, expressing EGFP under the control of the gG promoter. In PK15 cells infected with NIA-3 wild-type virus or with S3 recombinant virus, expression of gG PRV mRNA (or EGFP mRNA) under the control of the gG promoter in the presence of cycloheximide was detected by RT-PCR. This again indicates that some basal expression was produced in infected cells independently of IE180. This expression was augmented by IE180 protein in both plasmid transfections and viral infections.







Similar content being viewed by others
References
Bzik DJ, Preston CM (1986) Analysis of DNA sequences which regulate the transcription of herpes simplex virus immediate early gene 3: DNA sequences required for enhancer-like activity and response to trans-activation by a virion polypeptide. Nucleic Acids Res 14:929–943
Campbell ME, Preston CM (1987) DNA sequences which regulate the expression of the pseudorabies virus major immediate early gene. Virology 157:307–316
Deatly AJM, Ben Porat T (1985) Relation between the levels of mRNA abundance and kinetics of protein synthesis in pseudorabies virus-infected cells. Virology 143:558–568
Fernández A, Menéndez del Campo AM, Fernández S, Camacho A, Castro JM, Tabarés E (1999) Conversion of US3-encoded protein kinase gene from pseudorabies virus in a diploid gene located within inverted repeats by genetic recombination between the viral genome isomers. Virus Res 61:125–135
Gómez-Sebastián S, Tabarés E (2004) Negative regulation of herpes simplex virus type 1 ICP4 promoter by IE 180 protein of pseudorabies virus. J Gen Virol 85:2125–2130
Gould DJ, Chernajovsky Y (2004) Endogenous GATA factors bind the core sequence of the tetO and influence gene regulation with tetracycline system. Mol Ther 10:127–138
Ihara S, Feldman L, Watanabe S, Ben-Porat T (1983) Characterization of the immediate-early functions of pseudorabies virus. Virology 131:437–454
Jöns A, Mettenleiter TC (1997) Green fluorescent protein expressed by recombinant pseudorabies virus as an in vivo marker for viral replication. J Virol Methods 66:283–292
Jones C, Zhu F, Dhanwada KR (1993) Analysis of a herpes simplex virus 2 fragment from the open reading frame of the large subunit of ribonucleotide reductase with transcriptional regulatory activity. DNA Cell Biol 12:127–137
Klupp GB, Hengartner CJ, Mettenleiter TC, Enquist LW (2004) Complete, annotated sequence of the pseudorabies virus genome. J Virol 78:424–440
Kovacs F, Mettenleiter TC (1991) Firefly luciferase as a marker for herpesvirus (pseudorabies virus) replication in vitro and in vivo. J Gen Virol 72:2999–3008
Mackem S, Roizman B (1982) Structural features of the a gene 4, 0, and 27 promoter-regulatory sequences which confer a regulation on chimeric thymidine kinase genes. J Virol 68:4898–4909
McFerran JB, Dow C (1975) Studies on immunisation of pigs with the Bartha strain of Aujeszky’s disease virus. Res Vet Sci 19:17–22
Mettenleiter TC (2000) Aujeszky’s disease (pseudorabies) virus: the virus and molecular pathogenesis—state of the art, June 1999. Vet Res 31:99–115
Mettenleiter TC, Rauh I (1990) A glycoprotein gX-β-galactosidase fusion gene as a insertional marker for rapid identification of pseudorabies virus mutants. J Virol Methods 30:55–66
Muñoz AL, Prieto C, Tabarés E (2006) A comparison of enhanced green fluorescent protein expression induced by immediate-early cytomegalovirus (IE-CMV) and gG pseudorabies virus (gG-PRV) promoters, using pseudorabies virus amplicons as vectors. J Virol Methods 136:257–260
Nauwynck H, Glorieux S, Favoreel H, Pensaert M (2007) Cell biological and molecular characteristics of pseudorabies virus infections in cell cultures and in pigs with emphasis on the respiratory tract. Vet Res 38:229–241
O’Hare P, Goding CR (1988) Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell 52:435–445
Paul PS, Halbur P, Janke B, Joo H, Nawagitgul P, Singh J, Sorden S (2003) Exogenous porcine viruses. Curr Top Microbiol Immunol 278:125–183
Pellet PE, Roizman B (2007) The family of Herpesviridae: a brief introduction. In: Knipe DN, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (eds) Fields virology, 5th edn. Lippincott-Williams-Wilkins, Philadelphia, pp 2479–2499
Poffenberger KL, Tabarés E, Roizman B (1983) Characterization of a viable, noninverting herpes simplex virus 1 genome derived by insertion and deletion of sequences at the junction of components L and S. Proc Natl Acad Sci USA 80:2690–2694
Post LE, Mackem S, Roizman B (1981) Regulation of α genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with α gene promoters. Cell 24:555–565
Preston CM, Cordingley MG, Stow ND (1984) Analysis of DNA sequences which regulated the transcription of a herpes simplex virus immediate early gene. J Virol 50:708–716
Prieto J, Martin Hernández AM, Tabarés E (1991) Loss of pseudorabies virus thymidine kinase activity due a to single base mutation and amino acid substitution. J Gen Virol 72:1435–1439
Qiang P, Li XM, Jin ML, Peng CQ, Chen HC (2004) An approach to a FMD vaccine based on genetic engineered attenuated pseudorabies virus: one experiment using VP1 gene alone generates an antibody responds on FMD and pseudorabies in swine. Vaccine 22:2129–2136
Quinlan MP, Knipe DM (1985) Stimulation of expression of a herpes simplex virus DNA-binding protein by two functions. Mol Cell Biol 5:957–963
Rea TJ, Timmins JG, Long GW, Post LE (1985) Mapping and sequence of the gene for the pseudorabies virus glycoprotein which accumulates in the medium of infected cells. J Virol 54:21–29
Robbins AK, Watson RJ, Whealy ME, Hays WW, Enquist LW (1986) Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol 58:339–347
Roizman B, Knipe DM, Whitley RJ (2007) Herpes Simplex viruses. In: Knipe DN, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (eds) Fields virology, 5th edn. Lippincott-Williams-Wilkins, Philadelphia, pp 2501–2601
Shapira M, Homa FL, Glorioso JC, Levine M (1987) Regulation of the herpes simplex virus type 1 late (gamma 2) glycoprotein C gene: sequences between base pairs −34 to +29 control transient expression and responsiveness to transactivation by the products of the immediate early (alpha) 4 and 0 genes. Nucleic Acids Res 15:3097–3111
Silver S, Roizman B (1985) gamma 2-Thymidine kinase chimeras are identically transcribed but regulated a gamma 2 genes in herpes simplex virus genomes and as beta genes in cell genomes. Mol Cell Biol 5:518–528
Song CK, Enquist LW, Bartness TJ (2005) New developments in tracing neuronal circuits with herpesviruses. Virus Res 111:235–249
Tabarés E, Olivares I, Santurde G, García MJ, Martin E, Carnero ME (1987) African swine fever virus DNA: deletions and additions during adaptation to growth in monkey kidney cells. Arch Virol 97:333–346
Thomsen DR, Marotti KR, Palermo DP, Post LE (1987) Pseudorabies virus as a live virus vector for expression of foreign genes. Gene 57:261–265
Van Zijl M, Wensvoort G, Kluyver E, Hulst M, Gulden H, Gielkens A, Berns A, Moormann R (1991) Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera. J Virol 65:2761–2765
Van Oirschot GD, Gielskens ALJ, Moormann R, Berns A (1990) Marker vaccines, virus protein-specific antibody assay and the control of Aujeszky disease. Vet Microbiol 23:85–101
Viejo-Borbolla A, Muñoz AL, Tabarés E, Alcamí A (2010) Glycoprotein G from pseudorabies virus binds to chemokines with high affinity and inhibits their function. J Gen Virol 91:23–31
Vlcek C, Paces V, Schwyzer M (1989) Nucleotide sequence of the pseudorabies virus immediate early gene, encoding a strong transactivator protein. Virus Genes 2:335–346
Walboomers JMM, Ter Schegget J (1976) A new method for the isolation of herpes simplex virus type 2 DNA. Virology 79:256–258
Watson RJ, Colberg-Poley AM, Marcus-Sekura CJ, Carter BJ, Enquist LW (1983) Characterization of the herpes simplex virus type 1 glycoprotein D mRNA and expression of this protein in Xenopus oocytes. Nucleic Acids Res 11:1507–1522
Weir JP (2001) Regulation of herpes simplex expression. Gene 271:117–130
Xu G, Xu X, Li Z, He Q, Wu B, Sun S, Chen H (2004) Construction of recombinant pseudorabies virus expressing NS1 protein of Japanese encephalitis (SA14-14-2) virus and its safety and immunogenicity. Vaccine 22:1846–1853
Yamada S, Shimizu M (1994) Isolation and characterization of mutants of pseudorabies virus with deletion in the immediate-early regulatory gene. Virology 199:366–375
Acknowledgments
We are grateful to Esteban Domingo (CBM-UAM, Madrid, Spain) and Filip Lim for help with the English manuscript. This work was supported by a grant (SAF2004-08015) from the Ministerio de Educación y Ciencia (Spain), a grant PI070017 from Fondo Investigación Sanitaria (FIS) (Spain) and from The Wellcome Trust (UK) (075813/C/04/Z). A. L. Muñoz was supported by a fellowship from The Wellcome Trust (UK) (075813/C/04/Z).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muñoz, A.L., Torres, M., Martín, B. et al. Regulation of pseudorabies virus gG glycoprotein gene promoter independently of pseudorabies immediate early IE180 protein. Arch Virol 155, 515–523 (2010). https://doi.org/10.1007/s00705-010-0613-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-010-0613-4