Abstract
Rasagiline is a monoamine oxidase type-B inhibitor in development in Japan for Parkinson’s disease (PD). This open-label study evaluated the long-term safety and efficacy of rasagiline in Japanese patients with PD receiving levodopa. Patients were aged 30–79 years and had wearing-off or weakened effect. Patients received rasagiline 1 mg/day for 52 weeks. The primary objective was to evaluate safety. Secondary endpoints included MDS-UPDRS Part II and Part III total scores (ON-state) and change from baseline in mean daily OFF-time. An additional endpoint was the Parkinson’s Disease Questionnaire-39 (PDQ-39) Summary Index (SI) score. In total, 222 patients were enrolled; 52.3% had wearing-off phenomena. Treatment-emergent adverse events (TEAEs) were mostly mild or moderate and occurred in 83.3% of patients; 63.1% had drug-related TEAEs; and 21.2% had TEAEs resulting in discontinuation. Fall (16.7%), nasopharyngitis (14.0%), and dyskinesia (10.8%) were the most frequent TEAEs. Serious TEAEs were reported in 17.6% of patients, and led to discontinuation in 9.5%. At week 52 (last-observation-carried forward), the mean change from baseline in MDS-UPDRS Part III total score (ON-state) was − 7.6; the mean change from baseline in daily OFF-time was − 0.89 h in patients with wearing-off phenomena at the start of the run-in period. The mean change from baseline in PDQ-39 SI was − 0.64. No major safety issues were observed during this 52-week trial of rasagiline as an adjunct to levodopa in Japanese patients. Mean changes in MDS-UPDRS scores and daily OFF-time suggested that adjunctive rasagiline treatment with levodopa was efficacious, with efficacy maintained for at least 52 weeks.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease, that is second only in prevalence to Alzheimer’s disease and affects more than 300 per 100,000 individuals worldwide, most of whom are older than 50 years (Giovannoni et al. 2016; Pagano et al. 2016; Pringsheim et al. 2014). In Japan, 100–150 per 100,000 people suffer from the condition (Giovannoni et al. 2016), the prevalence of which is expected to increase, in parallel with the anticipated increase in the proportion of older people in the population.
Although the etiology of PD is complex, the key pathological feature is an early loss of dopaminergic neurons and reduced levels of dopamine in the nigrostriatal system (Kalia and Lang 2015). This is believed to be the main underlying cause of the characteristic motor symptoms of the condition, namely resting tremor, muscle rigidity, bradykinesia, and postural instability. Hence, most approved PD drugs [including levodopa, dopamine agonists, catechol-O-methyl transferase (COMT) inhibitors, and monoamine oxidase-B inhibitors (MAOB-Is)] are thought to alleviate symptoms by enhancing levels of dopamine or inhibiting its metabolism in specific brain regions. Drugs that do not act directly on the dopaminergic system (and which are less commonly used therapeutically) include anticholinergic drugs and amantadine (Schapira and Olanow 2008).
Levodopa is the naturally occurring immediate precursor of dopamine and is the most effective and commonly used drug for treating the motor symptoms of PD (Kalia and Lang 2015; Schapira 2007; Schapira and Olanow 2008; Tsouli and Konitsiotis 2010). However, long-term use can lead to motor complications, including wearing-off phenomena, which occur when the duration of therapeutic benefit of each levodopa dose gradually declines (Bhidayasiri et al. 2015; Chen et al. 2014; Schapira and Olanow 2008). Approximately 45% of Asian patients with PD develop wearing-off phenomena (Chen et al. 2014; Yoritaka et al. 2013). Japanese and international guidelines recommend that wearing-off is managed by adjusting the dose and/or formulation of levodopa or by use of adjunctive pharmacotherapy such as MAOB-Is (e.g., selegiline, rasagiline) and COMT inhibitors (Chen et al. 2014; DeMaagd and Philip 2015; Ferreira et al. 2013; Stowe et al. 2011; Takeda 2013). In Japan, istradefylline or zonisamide are also used adjunctively to levodopa for the management of wearing-off (Mizuno et al. 2010, 2013; Murata et al. 2007, 2015).
Rasagiline is a second-generation selective MAOB-I (Lecht et al. 2007) that is used both as monotherapy and in combination therapy in the treatment of PD (Chang et al. 2017). Based on findings from two phase 3 studies in patients with motor fluctuations during treatment with levodopa (Parkinson Study Group 2005; Rascol et al. 2005), rasagiline is currently indicated outside Japan as an adjunctive therapy for PD (Mitchell et al. 2005; Torkildsen et al. 2016).
We recently reported the results of two randomized, double-blind, placebo-controlled, 26-week studies of rasagiline therapy in Japanese patients with PD (Hattori et al. 2017, 2018). In combination with levodopa, rasagiline (0.5 or 1.0 mg/day) led to a reduction in mean daily OFF-time and an improvement in PD symptoms [assessed using the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)] (Kashihara et al. 2014) in Japanese patients with PD and wearing-off phenomena (Hattori et al. 2018). Furthermore, an improvement in quality of life [assessed using the Parkinson’s Disease Questionnaire (PDQ-39)] was observed and no new safety concerns were reported.
As PD is a chronic disease, long-term safety and efficacy data for rasagiline and levodopa combination therapy may provide useful insights for clinical practice. The two pivotal studies that evaluated the use of adjunctive rasagiline were 18- and 26-week studies (Parkinson Study Group 2005; Rascol et al. 2005). To our knowledge, no clinical studies have yet examined the long-term (> 26 weeks) safety and efficacy of rasagiline as an adjunctive treatment to levodopa in patients with PD. In the current study, we evaluated the safety and efficacy of long-term use of rasagiline 1 mg/day in combination with levodopa for up to 52 weeks in Japanese patients with PD.
Methods
Study design
This was a multi-center, open-label, phase 3 study designed to evaluate the safety and efficacy of long-term use of rasagiline (1 mg/day) as adjunctive therapy to levodopa, in Japanese patients with PD. The study began with a 2-week run-in period during which eligibility was determined. At the end of the run-in period, eligible patients received rasagiline 1 mg/day for up to 52 weeks. In principle, the dose of levodopa was to remain fixed from the start of the run-in period and throughout the treatment period. Change in levodopa dose was permitted, as deemed necessary by the investigators, but discontinuation was not allowed. Concomitant treatment with other PD agents (except selegiline) was permitted, provided that the dose did not change during the treatment period, wherever possible. Concomitant use of any antidepressants was prohibited. Patients used a diary to record ON-time without troublesome dyskinesia, ON-time with troublesome dyskinesia, OFF-time, and sleeping time, in 30-min intervals over 24 h during the 7 days preceding the weeks 0, 6, 10, 18, 26, 34, 42, and 52 visits.
The study was reviewed and approved by the Institutional Review Board at each of the participating study centers and was conducted in full compliance with the International Conference on Harmonization unified guidelines, the regulatory requirements of the region, and the ethical principles that have their origin in the Declaration of Helsinki. All patients provided written, informed consent. This study was registered with Clinical Trials.gov as NCT02337764.
Patients
Adult patients aged between 30 and 79 years, with a diagnosis of PD according to the UK Parkinson’s Disease Society Brain Bank diagnostic criteria were eligible for inclusion in the study. All patients had been continuously receiving levodopa plus a dopa decarboxylase inhibitor for at least 1 month prior to the start of the run-in period and were experiencing wearing-off phenomena or weakened effect (defined as initial efficacy at the start of therapy, with diminished effect over time) during treatment with levodopa. Exclusion criteria included: a modified Hoehn and Yahr stage (Goetz et al. 2004) of 5 (at either ON-time or OFF-time for patients with wearing-off phenomena); severe dyskinesia or unstable systemic disease; a Mini-Mental State Examination score of ≤ 24 at the start of the run-in period; and neurosurgical intervention for PD.
Endpoints and assessments
The primary endpoint was the incidence of treatment-emergent adverse events (TEAEs) during the 52-week treatment period. TEAEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) version 19.0 and were defined as any adverse events occurring after the first administration of rasagiline. Secondary endpoints were the MDS-UPDRS Part II (motor aspects of experiences of daily living) and Part III (motor examination) (Kashihara et al. 2014) total scores during ON-state, and mean daily OFF-time (averaged over 7 days before each visit) for patients with wearing-off phenomena at the start of the run-in period. Additional endpoints included MDS-UPDRS Part I (non-motor aspects of experiences of daily living) and Part IV (motor complications) total scores, and the PDQ-39 summary index and individual domain scores. MDS-UPDRS evaluations were performed by qualified and accredited investigators (Part IA, Part III and Part IV) or by patient self-assessment (Part IB and Part II); lower MDS-UPDRS scores indicate milder PD symptoms/signs (Kashihara et al. 2014). PDQ-39 is a patient self-reported questionnaire that assesses quality of life; lower scores indicate better quality of life (Jenkinson et al. 1997).
Patients were evaluated at weeks 3, 6, 10, 18, 26, 34, 42, and 52 during the treatment period. MDS-UPDRS scores were determined at weeks 0 and 6 and at every visit thereafter; PDQ-39 scores were determined at weeks 0, 26, and 52; TEAEs were recorded at every visit.
Statistical analysis
The safety analysis included data from patients who received at least one dose of the study drug. The last-observation-carried forward (LOCF) method was used to impute missing values for the 52-week timepoint for secondary efficacy endpoints and the additional endpoint of PDQ-39 scores. Descriptive statistics [means and two-sided 95% confidence intervals (CI)] were calculated for the changes from baseline in MDS-UPDRS total scores and mean daily OFF-time (for patients who had wearing-off phenomena during the run-in period) at each clinic visit. Similarly, descriptive statistics (means and two-sided 95% CI) were calculated for PDQ-39 scores. Taking feasibility into account, the planned number of patients to receive the study drug was set at 215.
Results
Patients
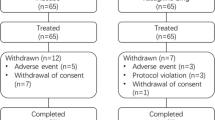
Of 241 patients providing informed consent, 222 patients were eligible to enter the study. Sixty patients (27%) discontinued before the end of the study; reasons for withdrawal were TEAEs (46 patients), voluntary withdrawal (12 patients), and lack of efficacy (2 patients) (Fig. 1).
At baseline (start of treatment), the mean age was 68 years, and the mean duration of PD was approximately 7 years (Table 1). The proportion of patients requiring a dose of levodopa of more than 300 mg/day was 77.8%. The mean duration of levodopa administration was 4.61 years. The proportion of patients with wearing-off phenomena was 52.3% with mean duration of wearing-off phenomena of approximately 3 years. The average MDS-UPDRS Part III (ON-state), II, I, and IV total scores at baseline were 28.8, 11.9, 7.8, and 3.0, respectively. Dopamine agonists were the most commonly used concomitant therapy for PD (Table 1).
Safety and tolerability
During the 52-week treatment period, 185 (83.3%) patients reported 599 TEAEs, and most of which were classified as mild to moderate (Table 2). Forty-seven patients (21.2%) discontinued the study due to TEAEs. Fall (16.7%), nasopharyngitis (14.0%), and dyskinesia (10.8%) were the most frequently occurring TEAEs (Table 3). The incidence of serious TEAEs was 17.6%; 9.5% of patients discontinued due to TEAEs (Table 2). During days 1–83 of rasagiline treatment, 116/222 patients (52.3%) had TEAEs, compared with 99/196 patients (50.5%) during days 84–167, 69/177 patients (39.0%) during days 168–251, 50/170 patients (29.4%) during days 252–335, and 19/164 patients (11.6%) after day 336. There were no deaths during the study.
Efficacy
After 6 weeks of adjunctive rasagiline, there was a greater than 5-point mean reduction in MDS-UPDRS Part III total score (ON-state); the improvement in score was maintained throughout the study (Fig. 2a). The mean change in MDS-UPDRS Part III total score (ON-state) from baseline to week 52 (LOCF) was − 7.6 (Table 4). By the first study visit (week 6), there was an approximate 1-point mean reduction in MDS-UPDRS Part II total score, followed by a small mean increase in score over the remaining weeks of the study (Fig. 2b). The mean change in MDS-UPDRS Part II total score from baseline to week 52 (LOCF) was 0.0 (Table 4). For MDS-UPDRS Part I and Part IV total scores, the mean changes from baseline to week 52 (LOCF) were 0.3 and − 0.2, respectively (Table 4).
Among 116 patients with wearing-off phenomena at the start of run-in period, the mean daily OFF-time at the end of the run-in period was 4.99 h (Table 1). After 6 weeks of adjunctive rasagiline, there was a mean reduction in daily OFF-time of approximately 1 h (Fig. 3). The reduction was maintained throughout the treatment period. The mean change in daily OFF-time per day from baseline to week 52 (LOCF) was − 0.89 h (Table 4).
At baseline, the mean PDQ-39 summary index was 18.06; after 52 weeks (LOCF) of adjunctive rasagiline, the mean change from baseline in Summary Index score was − 0.64 points. Mean scores for individual PDQ-39 domains were as follows: mobility [29.82 at baseline, change from baseline to week 52 (LOCF), − 0.97]; activities of daily living (22.79, − 2.21); emotional well-being (18.33, 0.37); stigma (14.02, − 1.59); social support (6.26, 0.31); cognition (21.94, 0.03); communication (10.41, 0.27); bodily discomfort (20.85, − 1.29) (Table 4).
Discussion
This single-arm, open-label, phase 3 study is the first evaluation of the safety and efficacy of long-term rasagiline as adjunctive therapy in patients with PD who had wearing-off phenomena or weakened effect during treatment with levodopa. TEAEs were generally mild to moderate, in line with the findings of the previous studies of rasagiline (Parkinson Study Group 2002, 2005; Rascol et al. 2005). Fall and nasopharyngitis were the most frequently occurring TEAEs. While these TEAEs were not reported as frequently in the PRESTO study or the LARGO study (Parkinson Study Group 2005; Rascol et al. 2005), the same TEAEs were reported in a 26-week study that investigated use of adjunctive rasagiline therapy in Japan (Hattori et al. 2018), as well as in other studies in Western populations (Olanow et al. 2009). In the PRESTO study, dyskinesia was reported more frequently in patients taking rasagiline 1 mg/day than in those taking 0.5 mg/day (Parkinson Study Group 2005). In the present study, the overall incidence of TEAEs was higher (52.3%) during days 1–83 after initiation of rasagiline than during subsequent periods; the incidence decreased over time. Administration of rasagiline for 52 weeks did not raise safety concerns in comparison with our previous study of adjunctive rasagiline for 26 weeks (Hattori et al. 2018). We highlight, however, that the rate of discontinuation due to TEAEs was 21.2% overall; as 18% had discontinued within the first 26 weeks, which aligns with results of the previous work [15.5% by week 26; (Hattori et al. 2018)], we attribute this seemingly high discontinuation rate to the long observation period in the present study.
The efficacy of long-term rasagiline in combination with levodopa was a secondary endpoint. To our knowledge, this was the first clinical trial to investigate the efficacy of 52 weeks of adjunctive rasagiline treatment in patients with PD, using MDS-UPDRS scores rather than the conventional UPDRS score. Treatment with adjunctive rasagiline led to improvements in motor function, as indicated by the sustained improvement in MDS-UPDRS Part III total score (ON-state). The mean improvement of − 7.6 points in MDS-UPDRS Part III total score (ON-state) from baseline to week 52 (LOCF) was considered to be clinically meaningful, based on the criteria proposed by Horvath et al. (Horvath et al. 2015). In patients with wearing-off phenomena at the start of the run-in period, treatment with rasagiline 1 mg/day led to a reduction in daily OFF-time versus baseline, which was maintained throughout the study; the mean change in daily OFF-time from baseline to week 52 (LOCF) was − 0.89 h. In our previous 26-week study, a greater mean decrease in daily OFF-time from baseline was observed in patients treated with rasagiline 1 mg/day (− 1.35) (Hattori et al. 2018). This difference between studies may be explained by the lack of an inclusion criterion in the present study defining daily OFF-time at baseline, which resulted in the enrollment of patients with shorter daily OFF-time in the present study relatively to the previous 26-week study (Hattori et al. 2018). Indeed, > 10% of patients with wearing-off phenomena had a daily OFF-time at baseline of less than 1 h, and in these patients, the change from baseline was understandably small, which may have affected the overall mean values. The present study was not designed to evaluate onset of efficacy in terms of reduction of daily OFF-time; however, a reduction was observed at the time of the first evaluation (6 weeks), similarly to our 26-week study and the PRESTO study (Hattori et al. 2018; Parkinson Study Group 2005).
Interpretations of the findings of this study are limited by the trial’s uncontrolled design. In the absence of a placebo arm, it is difficult to estimate the magnitude of effect that was attributable to the combination therapy. However, the mean changes from baseline at week 26 for the MDS-UPDRS Part III total score (ON-state) and daily OFF-time were similar to those determined in our previous study, in which there were significant differences versus the placebo group (Hattori et al. 2018), suggesting that the effects identified in the present study were drug-related. Since PD is a chronic disease and treatment is a life-long process, the present 52-week open-label study may not be sufficient to understand the long-term safety and efficacy of adjunctive rasagiline. Further longer term studies with treatment for > 52 weeks may be needed to advance our knowledge of this therapeutic strategy and optimize its clinical use.
In conclusion, rasagiline appears safe and tolerable as an adjunctive therapy in levodopa-treated Japanese patients with PD. Changes in MDS-UPDRS scores and daily OFF-time suggested that rasagiline treatment with levodopa was efficacious, with efficacy maintained for at least 52 weeks. These results support the use of rasagiline as a potential treatment option for Japanese patients with PD who develop wearing-off phenomena or weakened effect during treatment with levodopa.
References
Battisti WP et al (2015) Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med 163:461–464
Bhidayasiri R, Hattori N, Jeon B, Chen RS, Lee MK, Bajwa JA, Mok VC, Zhang B, Syamsudin T, Tan LC, Jamora RD, Pisarnpong A, Poewe W (2015) Asian perspectives on the recognition and management of levodopa ‘wearing-off’ in Parkinson’s disease. Expert Rev Neurother 15:1285–1297. https://doi.org/10.1586/14737175.2015.1088783
Chang Y, Wang LB, Li D, Lei K, Liu SY (2017) Efficacy of rasagiline for the treatment of Parkinson’s disease: an updated meta-analysis. Ann Med 49:421–434. https://doi.org/10.1080/07853890.2017.1293285
Chen W, Xiao Q, Shao M, Feng T, Liu WG, Luo XG, Chen XC, Xie AM, Liu CF, Liu ZG, Liu YM, Wang J, Chen SD (2014) Prevalence of wearing-off and dyskinesia among the patients with Parkinson’s disease on levodopa therapy: a multi-center registry survey in mainland China. Transl Neurodegener 3:26. https://doi.org/10.1186/2047-9158-3-26
DeMaagd G, Philip A (2015) Parkinson’s disease and its management: part 4: treatment of motor complications. Pharm Ther 40:747–773
Ferreira JJ, Katzenschlager R, Bloem BR, Bonuccelli U, Burn D, Deuschl G, Dietrichs E, Fabbrini G, Friedman A, Kanovsky P, Kostic V, Nieuwboer A, Odin P, Poewe W, Rascol O, Sampaio C, Schupbach M, Tolosa E, Trenkwalder C, Schapira A, Berardelli A, Oertel WH (2013) Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol 20:5–15. https://doi.org/10.1111/j.1468-1331.2012.03866.x
Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, Sormani MP, Thalheim C, Traboulsee A, Vollmer T (2016) Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord 9:S5–S48. https://doi.org/10.1016/j.msard.2016.07.003
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L, Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease (2004) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19:1020–1028. https://doi.org/10.1002/mds.20213
Hattori N, Takeda A, Takeda S, Nishimura A, Kitagawa T, Mochizuki H, Nagai M, Takahashi R (2017) Efficacy and safety of rasagiline monotherapy in Japanese patients with early Parkinson’s Disease: a phase 3, randomized, double-blind, placebo-controlled, multicenter study. Paper presented at the world congress of neurology (WCN), Kyoto, Japan
Hattori N, Takeda A, Takeda S, Nishimura A, Kato M, Mochizuki H, Nagai M, Takahashi R (2018) Efficacy and safety of adjunctive rasagiline in Japanese Parkinson’s disease patients with wearing-off phenomena: a phase 2/3, randomized, double-blind, placebo-controlled, multicenter study. Parkinsonism Relat Disord 53:21–27. https://doi.org/10.1016/j.parkreldis.2018.04.025
Horvath K, Aschermann Z, Acs P, Deli G, Janszky J, Komoly S, Balazs E, Takacs K, Karadi K, Kovacs N (2015) Minimal clinically important difference on the motor examination part of MDS-UPDRS. Parkinsonism Relat Disord 21:1421–1426. https://doi.org/10.1016/j.parkreldis.2015.10.006
Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N (1997) The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease Summary Index Score. Age Ageing 26:353–357
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Kashihara K, Kondo T, Mizuno Y, Kikuchi S, Kuno S, Hasegawa K, Hattori N, Mochizuki H, Mori H, Murata M, Nomoto M, Takahashi R, Takeda A, Tsuboi Y, Ugawa Y, Yamanmoto M, Yokochi F, Yoshii F, Stebbins GT, Tilley BC, Luo S, Wang L, LaPelle NR, Goetz CG, MDS-UPDRS Japanese Validation Study Group (2014) Official Japanese version of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale: validation against the original English version. Mov Disord Clin Pract 1:200–212. https://doi.org/10.1002/mdc3.12058
Lecht S, Haroutiunian S, Hoffman A, Lazarovici P (2007) Rasagiline—a novel MAO B inhibitor in Parkinson’s disease therapy. Ther Clin Risk Manag 3:467–474
Mitchell AJ, Benito-Leon J, Gonzalez JM, Rivera-Navarro J (2005) Quality of life and its assessment in multiple sclerosis: integrating physical and psychological components of wellbeing. Lancet Neurol 4:556–566. https://doi.org/10.1016/s1474-4422(05)70166-6
Mizuno Y, Hasegawa K, Kondo T, Kuno S, Yamamoto M, Japanese Istradefylline Study Group (2010) Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Mov Disord 25:1437–1443. https://doi.org/10.1002/mds.23107
Mizuno Y, Kondo T, Japanese Istradefylline Study Group (2013) Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov Disord 28:1138–1141. https://doi.org/10.1002/mds.25418
Murata M, Hasegawa K, Kanazawa I, Japan Zonisamide on P. D. Study Group (2007) Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology 68:45–50. https://doi.org/10.1212/01.wnl.0000250236.75053.16
Murata M, Hasegawa K, Kanazawa I, Fukasaka J, Kochi K, Shimazu R, Japan Zonisamide on P. D. Study Group (2015) Zonisamide improves wearing-off in Parkinson’s disease: a randomized, double-blind study. Mov Disord 30:1343–1350. https://doi.org/10.1002/mds.26286
Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, Langston W, Melamed E, Poewe W, Stocchi F, Tolosa E, Investigators AS (2009) A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 361:1268–1278. https://doi.org/10.1056/NEJMoa0809335
Pagano G, Ferrara N, Brooks DJ, Pavese N (2016) Age at onset and Parkinson disease phenotype. Neurology 86:1400–1407. https://doi.org/10.1212/WNL.0000000000002461
Parkinson Study Group (2002) A controlled trial of rasagiline in early Parkinson disease: the tempo study. Arch Neurol 59:1937–1943. https://doi.org/10.1001/archneur.59.12.1937
Parkinson Study Group (2005) A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol 62:241–248. https://doi.org/10.1001/archneur.62.2.241
Pringsheim T, Jette N, Frolkis A, Steeves TDL (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29:1583–1590. https://doi.org/10.1002/mds.25945
Rascol O, Brooks DJ, Melamed E, Oertel W, Poewe W, Stocchi F, Tolosa E, Group LS (2005) Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, lasting effect in adjunct therapy with rasagiline given once daily, study): a randomised, double-blind, parallel-group trial. Lancet 365:947–954. https://doi.org/10.1016/S0140-6736(05)71083-7
Schapira AH (2007) Treatment options in the modern management of Parkinson disease. Arch Neurol 64:1083–1088. https://doi.org/10.1001/archneur.64.8.1083
Schapira AH, Olanow CW (2008) Drug selection and timing of initiation of treatment in early Parkinson’s disease. Ann Neurol 64(Suppl 2):S47–S55. https://doi.org/10.1002/ana.21460
Stowe R, Ives N, Clarke CE, Handley K, Furmston A, Deane K, van Hilten JJ, Wheatley K, Gray R (2011) Meta-analysis of the comparative efficacy and safety of adjuvant treatment to levodopa in later Parkinson’s disease. Mov Disord 26:587–598. https://doi.org/10.1002/mds.23517
Takeda A (2013) Treatment & management guidelines 2011 for Parkinson disease. Rinsho Shinkeigaku 53:1346–1347
Torkildsen O, Myhr KM, Bo L (2016) Disease-modifying treatments for multiple sclerosis—a review of approved medications. Eur J Neurol 23:18–27. https://doi.org/10.1111/ene.12883
Tsouli S, Konitsiotis S (2010) How should we treat a patient with early Parkinson’s disease? Int J Clin Pract 64:1210–1219. https://doi.org/10.1111/j.1742-1241.2010.02371.x
Yoritaka A, Shimo Y, Takanashi M, Fukae J, Hatano T, Nakahara T, Miyamato N, Urabe T, Mori H, Hattori N (2013) Motor and non-motor symptoms of 1453 patients with Parkinson’s disease: prevalence and risks. Parkinsonism Relat Disord 19:725–731. https://doi.org/10.1016/j.parkreldis.2013.04.001
Acknowledgements
The authors would like to thank the patients who have participated in this study and their families, as well as the following study investigators in Japan: Satoe Shimoda at Ginza Internal Medicine Clinic, Yasuto Higashi at Himeji Central Hospital Clinic, Shinsuke Hamada at Hokuyukai Neurological Hospital, Tatsuya Hattori at Honmachi Clinic, Makio Takahashi at Japanese Red Cross Osaka Hospital, Kazuhito Tsuruta at Junwa Rehabilitation Foundation Junwakai Memorial Hospital, Nobuo Kohara at Kobe City Medical Center General Hospital, Naoyuki Kitagawa at Kohsei Chuo General Hospital, Hayato Yabe at Matsuyama Shimin Hospital, Masato Seike at Medical Corporation Bouchikai Izumino Hospital, Shunichiro Maruyama at Murakami Karindoh Hospital, Naoto Fujiki at National Hospital Organization Hokkaido Medical Center, Hiroshi Yamasaki at National Hospital Organization Hyogo-Chuo National Hospital, Keiji Chida at National Hospital Organization Iwate Hospital, Kazuko Hasegawa at National Hospital Organization Sagamihara National Hospital, Naomi Kanzato at Okinawa Prefectural Nanbu Medical Center and Children Medical Center, Takanori Hazama at Osaka General Medical Center, Chikako Kaneko and Soichi Katayama at Southern Tohoku Research Institute for Neuroscience Southern Tohoku Medical Clinic, Makoto Shiraishi at St. Marianna University School Of Medicine Hospital, Yoshihisa Tatsuoka at Tatsuoka Neurology Clinic, Toshihiko Suenaga at Tenri Hospital, Mutsumi Iijima at Tokyo Women’s Medical University Hospital, Akira Machida and Shoichiro Ishihara at Tsuchiura Kyodo General Hospital, Shigeki Arawaka at Yamagata University Hospital, and Hitoshi Yamada at Yokohama Neurology Clinic. The authors are also grateful to Tadayuki Kitagawa and Masafumi Kato, at Takeda Pharmaceutical Company, for reviewing the study protocol and the clinical study report, for the development of the statistical analysis plan, and for the statistical analysis. The authors would like to acknowledge the writing support of Ying Jean and Michael Simpson of FireKite, an Ashfield company, part of UDG Healthcare plc, during the development of this manuscript, which was funded by Takeda Pharmaceutical Co. Ltd., in compliance with Good Publication Practice 3 ethical guidelines (Battisti et al. 2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nobutaka Hattori has served on advisory boards for Hisamitsu Pharmaceutical Co Inc., Sumitomo Dainippon Pharma Co. Ltd, Ono Pharmaceutical Co. Ltd, Otsuka Pharmaceutical Co. Ltd, Novartis Pharma K.K., and Takeda Pharmaceuticals Co. Ltd; has performed corporate-sponsored research for Takeda Pharmaceutical Co. Ltd; has received honoraria from GlaxoSmithKline K.K., Nippon Boehringer Ingelheim Co. Ltd, FP Pharmaceutical Corporation, Sumitomo Dainippon Pharma Co. Ltd, Eisai Co. Ltd, Kissei Pharmaceutical Company, Nihon Medi-physics Co. Ltd, Kyowa Hakko-Kirin Co. Ltd, Novartis Pharma K.K, Biogen Japan Ltd, Acorda Therapeutics Inc., Otsuka Pharmaceutical Co. Ltd, Janssen Pharmaceutical K.K, Medtronic Japan Co. Ltd, Astellas Pharma Inc.; has received donations from Astellas Pharma Inc., Eisai Co. Ltd, MSD K.K., Daiichi Sankyo Co. Ltd, Novartis Pharma K.K., Takeda Pharmaceutical Co. Ltd, Nihon Medi-physics Co. Ltd, Sumitomo Dainippon Pharma Co. Ltd, Pfizer Japan Inc., Bayer Yakuhin Ltd, FP Pharmaceutical Corporation, Shionogi & Co. Ltd, MSD; and has received donations for the endowed research departments GlaxoSmithKline K.K., Nippon Boehringer Ingelheim, Co. Ltd, Sumitomo Dainippon Pharma Co. Ltd, Eisai Co. Ltd, Kissei Pharmaceutical Co., Nihon Medi-physics Co. Ltd, Kyowa Hakko-Kirin Co. Ltd, Medtronic Japan Co. Ltd, Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd, Mitsubishi Tanabe Pharma Co., Zaiho Co. Ltd, Asahi Kasei Medical Co. Ltd, MiZ Co. Ltd. Atsushi Takeda has served on advisory boards for AbbVie Inc., Kyowa Hakko-Kirin Co. Ltd, and Takeda Pharmaceutical Co. Ltd; has performed corporate-sponsored research for Hisamitsu Pharma Co. Inc., Meiji-Seika Pharma Co. Ltd, Pfizer Japan Inc.; and has received honoraria from AbbVie Inc., Sumitomo Dainippon Pharma Co. Ltd, Kyowa Hakko-Kirin Co. Ltd. Shinichi Takeda, Akira Nishimura, and Ryou Nakaya are employees of Takeda Pharmaceutical Co. Ltd. Hideki Mochizuki has served on advisory boards for Hisamitsu Pharmaceutical Co., Inc., Takeda Pharmaceutical Co. Ltd; has received honoraria from FP Pharmaceutical Corporation, Sumitomo Dainippon Pharma Co. Ltd, Nihon Medi-physics Co. Ltd, Kyowa Hakko-Kirin Co. Ltd, Novartis Pharma K.K., Otsuka Pharmaceutical Co. Ltd; has received donations from Nihon Medi-physics Co. Ltd, Sumitomo Dainippon Pharma Co. Ltd, Kyowa Hakko-Kirin Co. Ltd. Masahiro Nagai has served on an advisory board for Takeda Pharmaceuticals Co. Ltd; and has received honoraria from Novartis Pharma K.K. Ryosuke Takahashi is an employee of the Japan Agency for Medical Research and Development; has served on advisory boards for Kan Institute Co. Ltd and Sumitomo Dainippon Pharma Co. Ltd; has performed corporate-sponsored research for Novartis Pharma K.K. Co., Otsuka Pharmaceutical Co. Ltd, Pfizer Japan Inc., Takeda Pharmaceuticals Co. Ltd, Nippon Boehringer Ingelheim Co. Ltd, Sumitomo Dainippon Pharma Co. Ltd, Kyowa Hakko-Kirin Co. Ltd, Nihon Medi-Physics Co. Ltd, Mitsubishi Tanabe Pharma Co. and Konica Minolta Inc.; and has received honoraria from Sumitomo Dainippon Pharma Co Ltd., and FP Pharmaceutical Co.
Research involving human participants
This study was conducted with the highest respect for the study participants according to the protocol and the ethical principles that have their origin in the Declaration of Helsinki. The study was reviewed and approved by the Institutional Review Board at each of the participating study centers, and was conducted in full compliance with the International Conference on Harmonization unified guidelines and the regulatory requirements of the region.
Informed consent
This study was conducted in compliance with the regulations for consent as set forth in the Guidelines and Ordinance on Good Clinical Practice (GCP). All patients provided written, informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hattori, N., Takeda, A., Takeda, S. et al. Long-term safety and efficacy of adjunctive rasagiline in levodopa-treated Japanese patients with Parkinson’s disease. J Neural Transm 126, 289–297 (2019). https://doi.org/10.1007/s00702-018-1962-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-018-1962-5