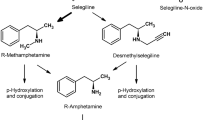
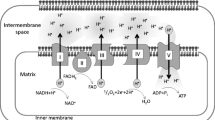
Abstract
In early 1920s, tyramine oxidase was discovered that metabolized tyramine and in 1933 Blaschko demonstrated that this enzyme also metabolized adrenaline, noradrenaline and dopamine. Zeller gave it the name monoamine oxidase (MAO) to distinguish it from the enzyme that oxidatively deaminated diamines. MAO was recognized as an enzyme of crucial interest to pharmacologists because it catalyzed the major inactivation pathway for the catecholamines (and, later, 5-hydroxytryptamine, as well). Within the few decade, the inhibitors of MAO were discovered and introduced for the treatment of depressive illness which was established clinically. However, the first clinical use exposed serious side effects, pharmacological interest in, and investigation of, MAO continued, resulting in the characterization of two forms, distinct forms, MAO-A and -B, and selective inhibitors for them. Selective inhibitors of MAO-B (selegiline, rasagiline and safinamide) have found a therapeutic role in the treatment of Parkinson’s disease and reversible inhibitors of MAO-A offered antidepressant activity without the serious side effects of the earlier nonselective MAO inhibitors. Subsequent molecular pharmacological have also generated the concept of neuroprotection, reflecting the possibility of slowing, halting and maybe reversing, neurodegeneration in Parkinson’s or Alzheimer’s diseases. Increased levels of oxidative stress through the accumulation of iron in the Parkinsonian and Alzheimer brains has been suggested to be critical for the initiation and progress of neurodegeneration. Selective inhibition of brain MAO could contribute importantly to lowering such stress, preventing the formation of hydrogen peroxide. Interaction of Iron with hydrogen peroxide and lead to Fenton reaction and production of the most reactive radical, namely hydroxyl radical. There are complex interactions between free iron levels in brain and MAO, and cascade of neurotoxic events may have practical outcomes for depressive disorders and neurodegenerative diseases. As consequence recent novel therapeutic drugs for neurodegenerative diseases has led to the development of multi target drugs, that possess selective brain MAO A and B inhibitory moiety, iron chelating and antioxidant activities and the ability to increase brain levels of endogenous neurotrophins, such as BDNF, GDNF VEGF and erythropoietin and induce mitochondrial biogenesis.





Similar content being viewed by others
References
Aarsland D, Larsen JP, Cummins JL, Laake K (1999) Prevalance and clinical correlates of psychosis symptoms in Parkinson’s diseases; a community-based study. Arch Neurol 56:595–601
Adolfsson R, Gottfries CG, Oreland L, Wiberg A, Winblad B (1980) Increased activity of brain and platelet monoamine oxidase in dementia of Alzheimer type. Life Sci 27:1029–1034
Amit T, Avramovich-Tirosh Y, Youdim MB, Mandel S (2008) Targeting multiple Alzheimer’s disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J 22(5):1296–1305
Amit T, Bar-Am O, Mechlovich D, Kupershmidt L, Youdim MBH, Weinreb O (2017) The novel multitarget iron chelating and propargylamine drug M30 affects APP regulation and processing activities in Alzheimer’s disease models. Neuropharmacology 123:359–367
Amrein R, Martin JR, Cameron AM (1999) Moclobemide in patients with dementia and depression. Adv Neurol 80:509–519
Angst J, Amrien R, Stabl M (1995) Moclobemide and tricyclic antidepressants in severe depression: meta analysis and prospective studies. Clin Psychpharmacol 15:16S–24S
Avramovich-Tirosh Y, Amit T, Bar-Am O, Zheng H, Fridkin M, Youdim MB (2007) Therapeutic targets and potential of the novel brain-permeable multifunctional iron chelator-monoamine oxidase inhibitor drug, M-30, for the treatment of Alzheimer’s disease. J Neurochem 100:490–502
Bar-Am O, Weinreb O, Amit T, Youdim MB (2005) Regulation of Bcl-2 family proteins, neurotrophic factors, and APP processing in the neurorescue activity of propargylamine. FASEB J 19:1899–1901
Bar-Am O, Amit T, Kupershmidt L, Aluf Y, Mechlovich D, Kabha H, Danovitch L, Zurawski VR, Youdim MB, Weinreb O (2015) Neuroprotective and neurorestorative activities of a novel iron chelator-brain selective monoamine oxidase-A/monoamine oxidase-B inhibitor in animal models of Parkinson’s disease and aging. Neurobiol Aging 36(3):1529–1542
Bech P (1993) Depressive syndrome in Parkinson’s disease: clinical manifestation. In: Mental dysfunction in Parkinson’s disease. Vrije, Amsterdam, pp 314–324
Ben Shachar D, Kahana N, Kampel V, Warshawsky A, Youdim MB (2004) Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lession in rats. Neuropharmacology 46(2):254–263
Ben-Shachar D, Eshel G, Finberg JP, Youdim MB (1991) The iron chelator desferrioxamine (Desferal) retards 6-hydroxydopamine-induced degeneration of nigrostriatal dopamine neurons. J Neurochem 56(4):1441–1444
Bette S, Shpiner DS, Singer C, Moore H (2018) Safinamide in the management of patients with Parkinson’s disease not stabilized on levodopa: a review of the current clinical evidence. Ther Clin Risk Manag 14:1737–1745
Bieck PR, Antonin KH, Schmidt E, Wurhrich I, Haefely WE (1988) Clinical pharmacology of reversible monoamine oxidase inhibitors. Clin Neuropharmacol 16(Suppl. 2):S34–S41
Binde CD, Tvete IF, Gåsemyr J, Natvig B, Klemp M (2018) A multiple treatment comparison meta-analysis of monoamine oxidase type B inhibitors for Parkinson’s disease. Br J Clin Pharmacol 84(9):1917–1927
Birkmayer W, Riederer P (1975) Biochemical post-mortem findings in depressed patients. J Neural Transm 37(2):95–109
Birkmayer W, Riederer P (1986) Biological aspects of depression in Parkinson’s disease. Psychopathology 19(Suppl 2):58–61
Birkmayer W, Riederer P, Youdim MB, Linauer W (1975) The potentiation of the anti akineti effect after l-dopa treatment by an inhibitor of MAO-B, deprenil. J Neural Transm 36(3–4):303–326
Birkmayer W, Riederer P, Ambrozi L, Youdim MB (1977) Implications of combined treatment with ‘Madopar’ and l-deprenil in Parkinson’s disease. A long-term study. Lancet 26(8009):439–443 1(
Bridge TP, Soldo BJ, Phelps BH, Wise CD, Rancak MJ, Wyatt RJ (1985) Platelet monoamine oxidase activity demographic characteristics contribute to enzyme activity variability. J Gerontol 40:23–28
Carradori S, Secci D, Petzer JP (2018) MAO inhibitors and their wider applications: a patent review. Expert Opin Ther Pat 28(3):211–226
Cesura AM, Pletscher A (1992) The new generation of monoamine oxidase inhibitors. Prog Drug Res 38:171–279
Chan-Palay V (1992) Depression and dementia of Alzheimer’s type: a role for moclobemide. Psychopharmacology 106:5137–5514
Collins GGS, Sandler M, Williams ED, Youdim MBH (1970) Multiple forms of human brain monoamine oxidase. Nature 225:817–820
Coppen A (1967) The biochemistry of affective disorders. Br J Psychiatry 113(504):1237–1264
Corbineau S, Breton M, Mialet-Perez J, Costemale-Lacoste JF (2017) Major depression and heart failure: interest of monoamine oxidase inhibitors. Int J Cardiol 247:1–6
Da Prada M, Keller H, Keller R et al (1981) Ro 11-1163, a specific short acting MAO inhibitor with antidepressant properties. In: Monoamine oxidase. Basic and clinical frontiers. Excerpta Medica, Amsterdam, pp 183–196
Da Prada M, Zürcher G, Wüthrich I, Haefely WE (1988) On tyramine, food, beverages and the reversible MAO inhibitor moclobemide. J Neural Transm Suppl 26:31–56
Deshwal S, Di Sante M, Di Lisa F, Kaludercic N (2017) Emerging role of monoamine oxidase as a therapeutic target for cardiovascular disease. Curr Opin Pharmacol 33:64–69
Domschke K, Tidow N, Schwarte K, Ziegler C, Lesch KP, Deckert J, Arolt V, Zwanzger P, Baune BT (2015) Pharmacoepigenetics of depression: no major influence of MAO-A DNA methylation on treatment response. J Neural Transm (Vienna) 122(1):99–108
Edmondson DE, Binda C (2018) Monoamine oxidases. Subcell Biochem 87:117–139
Erfurth AB. Back T (1999) Severe therapy refractory depression as initial mainstream of Parkinson’s disease. Psychiatr Pract 26:46–47
Finberg JPM, Tenne M, Youdim MBH (1981) Tyramine antagonistic properties of AGN-1135, and irreversible inhibitor of monoamine oxidase B. Br J Pharmacol 73:65–70
Gal S, Zheng H, Fridkin M, Youdim MB (2005) Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases. In vivo selective brain monoamine oxidase inhibition and prevention of MPTP-induced striatal dopamine depletion. J Neurochem 95:79–88
Gal S, Zheng H, Fridkin M, Youdim MB (2010) Restoration of nigrostriatal dopamine neurons in post-MPTP treatment by the novel multifunctional brain-permeable iron chelator-monoamine oxidase inhibitor drug, M30. Neurotoxic Res 17:15–27
Glezer S, Finberg JP (2003) Pharmacological comparison between the actions of methamphetamine and 1-aminoindan stereoisomers on sympathetic nervous function in rat vas deferens. Eur J Pharmacol 472(3):173–177
Golko-Perez S, Amit T, Bar-Am O, Youdim MB, Weinreb O (2017) A novel iron chelator-radical scavenger ameliorates motor dysfunction and improves life span and mitochondrial biogenesis in SOD1G93A ALS Mice. Neurotoxic Res 31(2):230–244
Green AR, Grahame-Smith DG (1975) Handbook of psychopharmacology, vol, 3. Plenum, New York, pp 350–388
Green AR, Youdim MBH (1976) Use of animal models to study the action of monoamine oxidase inhibitors. In: Knight J (ed) Monoamine oxidase and its inhibition. Ciba Foundation Symposium No.39. Elsevier, Amsterdam, pp 231–246
Green AR, Mitchell BD, Tordoff AF, Youdim MB (1977) Evidence for dopamine deamination by both type A and type B monoamine oxidase in rat brain in vivo and for the degree of inhibition of enzyme necessary for increased functional activity of dopamine and 5-hydroxytryptamine. Br J Pharmacol 60(3):343–349
Haefely W, Burkard WP, Cesura AM et al (1992) Biochemistry and pharmacology of moclobemide, a prototype RIMA. Psychopharmacology 106:56–514
Haefely W, Burkard WP, Cesura A et al (1993) Pharmacology of moclobemide. Clin Neuropharmacol 13(suppl 2):58–518
Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP (1996) Allelic variation of human serotonin transporter gene expression. J Neurochem 66(6):2621–2624
Jansen ENH. Ballering LAP (1999) Combined and selective monoamine oxidase inhibition in the treatment of depression in Parkinson’s disease. Adv Neurol 80:505–509
Janssen PA, Leysen JE, Megens AA, Awouters FH (1999) Does phenylethylamine act as an endogenous amphetamine in some patients? Int J Neuropsychopharmacol 2(3):229–240
Jellinger K, Kienzl E, Rumpelmair G, Riederer P, Stachelberger H, Ben-Shachar D, Youdim MB (1992) Iron-melanin complex in substantia nigra of parkinsonian brains: an X-ray microanalysis. J Neurochem 59(3):1168–1171
Johnston P (1968) Some observations upon a new inhibitor of monoamine oxidas in brain tissue. Biochem Pharmacol 17:1285–1297
Jones AB, Pare CM, Nicholson WJ, Price K (1972) Stacey RSBrain amine concentrations after monoamine oxidase inhibitor administration. Br Med J 1(5791):17–19
Kalir A, Sabbagh A, Youdim MBH (1981) Selective acetylenic “suicide” and reversible inhibitors of monoamine oxidase A and B. Br J Pharmacol 73:55–64
Knoll J, Magyar K (1972) some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv Biochem Psychopharmacol 5:393–408
Konradi C, Riederer P, Jellinger K, Denney R (1987) Cellular action of MAO inhibitors. J Neural Transm Suppl 25:15–25
Konradi C, Svoma E, Jellinger K, Riederer P, Denney R, Thibault J (1988) Topographic immunocytochemical mapping of monoamine oxidase-A, monoamine oxidase-B and tyrosine hydroxylase in human post mortem brain stem. Neuroscience 26(3):791–802
Konradi C, Kornhuber J, Sofic E, Heckers S, Riederer P, Beckmann H (1992) Variations of monoamines and their metabolites in the human brain putamen. Brain Res 8(2):285–290 579
Korn A, Da Prada M, Raffesberg M, Allen S, Casic S (1988) Tyramine pressor effect in man studies with moclobemide a novel reversible monoamine oxidase inhibitor. J Neural Transm Suppl 26:57–72
Korpelainen JT. Hiltunen P. Myllyla VV (1998) Moclobemide –induced hypersexuallity in patients with stroke and Parkinson’s disease. Clin Neuropharmacol 21:251–254
Kuhn DM, Wolf WA, Youdim MB (1986) Serotonin neurochemistry revisited: a new look at some old axioms. Neurochem Int 8(2):141–154
Kulisevsky J. Pascula-Sedano B (1999) Parkinson’s disease and cognition. Neurologia 14(Suppl. 1):72–81
Kupershmidt L, Weinreb O, Amit T, Mandel S, Bar-Am O, Youdim MB (2011) Novel molecular targets of the neuroprotective/neurorescue multimodal iron chelating drug M30 in the mouse brain. Neuroscience 189:345–358
Kupershmidt L, Amit T, Bar-Am O, Youdim MB, Weinreb O (2012a) Multi-target, neuroprotective and neurorestorative M30 improves cognitive impairment and reduces Alzheimer’s-like neuropathology and age-related alterations in mice. Mol Neurobiol 46(1):217–220
Kupershmidt L, Amit T, Bar-Am O, Youdim MB, Weinreb O (2012b) The novel multi-target iron chelating-radical scavenging compound M30 possesses beneficial effects on major hallmarks of Alzheimer’s disease. Antioxid Redox Signal 17(6):860–877
Lang AE, Lozano AM (1998a) Parkinson’s disease. Second of two parts. The New England journal of medicine 339:1130–1143
Lang AE, Lozano AM (1998b) Parkinson’s disease. First of two parts. N Engl J Med 339:1044–1053
Less AJ, Shaw KM, Kohout LJ, Sandler M, Youdim MBH (1977) Deprenyl in Parkinson’s disease. Lancet 2(8042):791–795
Lotharius J, Brundin P (2002) Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 3:932–942
Mann JJ. Gershon D (1980) l-Deprenyl, a monoamine oxidase type B inhibitor in endogenous depression. Life Sci 26:877–882
Mann JJ, Aarons SF, Wilner PJ, Keilp JG, Sweeney JA, Pearlstein T, Frances AJ, Kocsis JH, Brown RP (1989) A controlled study of antidepressant efficacy and side effects of l-deprenyl. A selective monoamine oxidase inhibitor. Arch Gen Psychiatry 46:45–50
Mendlewicz J, Youdim MBH (1978) Anti-depressant potentiation of 5-hydroxytryptophan by l-deprenyl, a monoamine oxidase type B inhibitor. J Neural Transm 43:279–286
Mendlewicz J. Youdim MBH (1979) Anti-depressant potentiation of 5-hydroxytryptophan by l-deprenyl in affective disorder. J Affect Disord 2:137–146
Mendlewicz J. Youdim MBH (1983) l-Deprenyl, a selective monoamine oxidase type B inhibitor in the treatment of depression. A double blind examination. Br J Psychiatry 142:507–511
Morphy R, Kay C, Rankovic Z (2004) From magic bullets to designed multiple ligands. Drug Discov Today 9:641–651
Mössner R, Henneberg A, Schmitt A, Syagailo Y, Grässle M, Hennig T, Simantov R, Gerlach M, Riederer P, Lesch KP (2001) Allelic variation of serotonin transporter expression is associated with depression in Parkinson’s disease. Mol Psychiatry 6(3):350–352
Müller T (2017) Pharmacokinetic drug evaluation of safinamide mesylate for the treatment of mid-to-late stage Parkinson’s disease. Expert Opin Drug Metab Toxicol 13(6):693–699
Murphy R, Rankovic Z (2005) Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem 48:6523–6543
Murphy DL, Wright C, Buchsbaum M, Nichols A, Costa JL, Wyatt RJ (1976) Platelet and plasma amine oxidase activity in 680 normals: sex and age differences and stability over time. Biochem Med 16:254–265
Naoi M, Maruyama W, Shamoto-Nagai M (2018) Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: from neurotransmitter imbalance to impaired neurogenesis. J Neural Transm (Vienna) 125(1):53–66
O’Carroll AM, Fowler CJ, Phillips JP, Tobia I, Tipton KF (1983) The deamination of dopamine by human brain monoamine oxidase. Arch Pharmacol 322:198–223
Paykel E, Youdim MBH (eds) (1993) Monoamine oixidase inhbitors as anti depressants. Raven press, New York
Pimentel LS, Allard S, Do Carmo S, Weinreb O, Danik M, Hanzel CE, Youdim MB, Cuello AC (2015) The multi-target drug M30 shows pro-cognitive and anti-inflammatory effects in a rat model of Alzheimer’s disease. J Alzheimers Dis 47(2):373–383
Poewe W, Luginger E (1999) Depression in Parkinson’s disease: impediment to recognition and treatment option. Neurology 52:S2–S6
Priest RG (1992) Moclobemide, a range of opportunities. Psychopharmacology 106:5140–5142
Reif A, Weber H, Domschke K, Klauke B, Baumann C, Jacob CP, Ströhle A, Gerlach AL, Alpers GW, Pauli P, Hamm A, Kircher T, Arolt V, Wittchen HU, Binder EB, Erhardt A, Deckert J (2012) Meta-analysis argues for a female-specific role of MAOA-uVNTR in panic disorder in four European populations. Am J Med Genet B Neuropsychiatr Genet 159B(7):786–793
Reynolds GP, Riederer P, Sandler M, Jellinger K, Seemann D (1978) Amphetamine and 2-phenylethylamine in post-mortem Parkinsonian brain after (−)deprenyl administration. J Neural Transm 43(3–4):271–277
Reynolds GP, Riederer P, Sandler M (1979) 2-Phenylethylamine and amphetamine in human brain: effects of l-deprenyl in Parkinson’s disease [proceedings]. Biochem Soc Trans 7(1):143–145
Ricken R, Ulrich S, Schlattmann P, Adli M (2017) Tranylcypromine in mind (Part II): review of clinical pharmacology and meta-analysis of controlled studies in depression. Eur Neuropsychopharmacol 27(8):714–731
Riederer P, Laux G (2011) MAO-inhibitors in Parkinson’s disease. Exp Neurobiol 20(1):1–17
Riederer P, Müller T (2018) Monoamine oxidase-B inhibitors in the treatment of Parkinson’s disease: clinical-pharmacological aspects. J Neural Transm (Vienna) (in press)
Riederer P, Youdim MBH (1986) Human brain monoamine oxidase activity and monoamine metabolism in Parkinsonian patients treated with l deprenyl. J Neurochem 45:1349 56
Riederer P, Reynolds GP, Youdim MBH, Jellinger K (1982) In vitro tests of MAO inhibitors in human brain tissue: chemical structure and pharmacological action. In: Kamijo K, Usdin E, Nagatsu T (eds) Monoamine oxidase. Basic and clinical frontiers. Excerpta Medica. International congress series 564. Proceedings of a symposium held in Hakone, Japan, July 1981
Riederer P. Konradi C, Schay V, Kienzl E, Birkmayer W, Youdim MBH (1986) Location of MAOA and MAOB in human brain. A step in understanding the therapeutic action of ldeprenyl. Adv Neurol 45:11119
Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB (1989) Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 52(2):515–520
Riederer P, Lachenmayer L, Laux G (2004a) Clinical applications of MAO-inhibitors. Curr Med Chem 11(15):2033–2043
Riederer P, Danielczyk W, Grunblatt E (2004b) Monoamine oxidase-B inhibition in Alzheimer’s disease. Neurotoxicology 25:271–277
Sagi Y, Weinstock M, Youdim MB (2003) Attenuation of MPTP-induced dopaminergic neurotoxicity by TV3326, a cholinesterase-monoamine oxidase inhibitor. J Neurochem 86:290–297
Schapira AH (2011) Monoamine oxidase B inhibitors for the treatment of Parkinson’s disease: a review of symptomatic and potential disease-modifying effects. CNS Drugs 25(12):1061–1071
Schildkraut JJ (1967) The catecholamine hypothesis of affective disorders. A review supporting evidence. Int J Psychiatry 4(3):203–217
Schmauss M (2002) Kontrolluntersuchungen. In: Riederer P, Laux G (eds) Pöldinger (Hrsg) Neuro-Psychopharmaka, Band 3: Antidepressiva, Phasenpophylaktika und Stimmungsstabilisier 2, neubearbeitete Auflage. Springer, Wien, pp 538–539
Serra-Mestres J, Ring HA (1999) Vulnerability to emotional negative stimuli in Parkinson’s disease: an investigation using the emotional stroop task. Neuropychiatry Neuropychol Behav Neurol 12:52–57
Sieradzan K, Chanon S, Stern G, Youdim MBH (1995) The therapeutic potential of moclobemide, a reversible selective monoamine oxidase A inhibitor in Parkinson’s disease. Clin Psychopharmacol 15:51S–60S
Sterling J, Herzig Y, Goren T, Finkelstein N, Lerner D, Goldenberg W, Miskolczi I, Molnar S, Rantal F, Tamas T, Toth G, Zagyva A, Zekany A, Finberg J, Lavian G, Gross A, Friedman R, Razin M, Huang W, Krais B, Chorev M, Youdim MB, Weinstock M (2002) Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer’s disease. J Med Chem 45:5260–5279
Sternic N. kacar A. Filipovic S. Svetel M, Kostic VS (1998) The therapeutic effect of moclobemide, a reversible selective monoamine oxidase A inhibitor in Parkinson’s disease. Clin Neuropharmacol 21:93–96
Stinton C, McKeith I, Taylor JP, Lafortune L, Mioshi E, Mak E, Cambridge V, Mason J, Thomas A, O’Brien JT (2015) Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry 172(8):731–742
Szeleny I (ed) (1993) Inhibitors of monoamine oxidase B. Boston: Birkhäuser. Parkinson Study Group. Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N Eng J Med 28:176–183
Szökő É, Tábi T, Riederer P, Vécsei L, Magyar K (2018) Pharmacological aspects of the neuroprotective effects of irreversible MAO-B inhibitors, selegiline and rasagiline, in Parkinson’s disease. J Neural Transm (Vienna)
Teixeira FG, Gago MF, Marques P, Moreira PS, Magalhães R, Sousa N, Salgado AJ (2018) Safinamide: a new hope for Parkinson’s disease? Drug Discov Today 23(3):736–744
Ulrich S, Ricken R, Adli M (2017) Tranylcypromine in mind (Part I): review of pharmacology. Eur Neuropsychopharmacol 227(8):697–713
Vallldeoriola F, Molinuevo J (1999) Therapy of behavioural disorders in Parkinson’s disease. Biomed Parmamacother 53:149–153
Van Praag HM (1974) Therapy-resistant depressions. Biochemical and pharmacological considerations. Contributions to biochemistry. Pharmakopsychiatr Neuropsychopharmakol 7(2):88–98
Van Praag HM (1993) Serotonin and affective psychopathology in Parkinson’s disease. A psychological consequence. In: Mental Dysfunction in Parkinson’s disease. Vrije, Amsterdam, pp 335–350
Van der Schyf CJ, Gal S, Geldenhuys WJ, Youdim MB (2006a) Multifunctional neuroprotective drugs targeting monoamine oxidase inhibition, iron chelation, adenosine receptors, and cholinergic and glutamatergic action for neurodegenerative diseases. Expert Opin Investig Drugs 15:873–886
Van der Schyf CJ, Geldenhuys WJ, Youdim MB (2006b) Multifunctional neuroprotective drugs for the treatment of cognitive and movement impaired disorders, including Alzheimer’s and Parkinson’s diseases. Drugs Future 31:447–460
Weinreb O, Mandel S, Bar-Am O, Yogev-Falach M, Avramovich-Tirosh Y, Amit T, Youdim MB (2009) Multifunctional neuroprotective derivatives of rasagiline as anti-Alzheimer’s disease drugs. Neurotherapeutics 6(1):163–174
Weinreb O, Amit T, Mandel S, Kupershmidt L, Youdim MB (2010) Neuroprotective multifunctional iron chelators: from redox-sensitive process to novel therapeutic opportunities. Antioxid Redox Signal 13(6):919–949
Weinreb O, Amit T, Bar-Am O, Youdim MB (2012) Ladostigil: a novel multimodal neuroprotective drug with cholinesterase and brain-selective monoamine oxidase inhibitory activities for Alzheimer’s disease treatment. Curr Drug Targets 13(4):483–494
Weinstock M, Bejar C, Wang RH, Poltyrev T, Gross A, Finberg JP, Youdim MB (2000) TV 3326, a novel neuroprotective drug with cholinesterase and monoamine oxidase inhibitory activities for the treatment of Alzheimer´s disease. J Neural Transm Suppl 60:157–169
Weinstock M, Kirschbaum-Slager N, Lazarovici P, Bejar C, Youdim MB, Shoham S (2001) Neuroprotective effects of novel cholinesterase inhibitors derived from rasagiline as potential anti-Alzheimer drugs. Ann NY Acad Sci 939:148–161
Weinstock M, Gorodetsky E, Wang RH, Gross A, Weinreb O, Youdim MB (2002a) Limited potentiation of blood pressure response to oral tyramine by brain-selective monoamne oxidase A-B inhibitor, TV-3326 in conscious rabbits (1). Neurpharmacology 43(6):999–1005
Weinstock M, Polytrev T, Bejar C, Youdim MB (2002b) Effect of TV 3326, a novel monoamine-oxidase cholinesterase inhibitor, in rat models of anxiety and depression. Psychopharmacology 160(3):318–324
Weinstock M, Gorodetsky E, Poltyrev T, Gross A, Sagi Y, Youdim MB (2003) A novel cholinesterase and brain-selective monoamine oxidase inhibitor for the treatment of dementia comorbid with depression and Parkinson´s disease. Prog Neuropsychopharmacol Biol Psychiatry 27(4):555–561
Yang HY, Neff NH (1973) Beta-phenylethylamine: a specific substrate for type B monoamine oxidase of brain. J Pharmacol Exp Ther 187(2):365–371
Yogev-Falach M, Bar-Am O, Amit T, Weinreb O, Youdim MB (2006) A multifunctional, neuroprotective drug, ladostigil (TV3326), regulates holo-APP translation and processing. FASEB J 20(12):2177–2179
Youdim MBH (ed) (1995) Reversible and selective inhibitors of monoamine oxidase: new findings. Acta Psychiatr Scand 91:1–43
Youdim MB, Buccafusco JJ (2005a) Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol Sci 26:27–35
Youdim MB, Buccafusco JJ (2005b) CNS targets for multi-functional drugs in the treatment of Alzheimer’s and Parkinson’s diseases. J Neural Transm 112:519–537
Youdim MHB, Riederer P (1993) Dopamine metabolism and neurotransmission in primate brain in relationship to monoamine oxidase A and B inhibition. J Neural Transm Gen Sect 91(2–3):181–195
Youdim MBH, Sourkes TL (1965) The effect of heat, pH and riboflavin deficiency on monoamine oxidase activity. Can J Biochem 43:1305–1318
Youdim MB, Weinstock M (2001) Molecular basis of neuroprotective activities of rasagaline and the anti-Alzheimer drug TV3326 [(N-prpargyl-(3R) aminoindan-5YL)-ethyl methyl carbamate]. Cell Mol Neurobiol 21(6):555–573
Youdim MB, Weinstock M (2002) Novel neuroprotective anti-Alzheimer drugs with anti-depressant activity derived from the anti-Parkinson drug, rasagiline. Mech Ageing Dev 123(8):1081–1086
Youdim MBH, Collins GGS, Sandler M, Bevan-Jones E, Nicholson A (1972) Human brain monoamine oxidase, multiple forms and selective inhibitors. Nature 236:225–228
Youdim MBH. Green AR. Grahame-Smith DG (1976) The role of 5-hydroxytryptamine, dopamine and MAO in the production of hyperactivity. In: Advances in Parkinsonism Editiones Roche, Basel, pp 155–163
Youdim MBH, Finberg JPM, Tipton KF (1988a) Monoamine oxidase. In: Trendelengurg U, Weiner U (eds) Catecholamine II. Springer, Berlin, pp 119–192
Youdim MBH, Da Prada M, Amrein R (eds) (1988b) The cheese effect and new reversible MAO-A inhibitors. J Neural Transm Suppl 26:31–56
Youdim MB, Stephenson G, Ben Shachar D (2004) Ironing iron out in Parkinson’s disease and other neurodegenerative diseases with iron chelators: a lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Ann N Y Acad Sci 1012:306–325
Youdim MB, Fridkin M, Zheng H (2005a) Bifunctional drug derivatives of MAO-B inhibitor rasagiline and iron chelator VK-28 as a more effective approach to treatment of brain ageing and ageing neurodegenerative diseases. Mech Ageing Dev 126:317–326
Youdim MB, Am B, Yogev-Falach O, Weinreb M, Maruyama O, Naoi WM, Amit T (2005b) Rasagiline: neurodegeneration, neuroprotection, and mitochondrial permeability transition. J Neurosci Res 79:172–179
Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR (2004) Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5:863–873
Zheng H, Gal S, Weiner LM, Bar-Am O, Warshawsky A, Fridkin M, Youdim MB (2005a) Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases: in vitro studies on antioxidant activity, prevention of lipid peroxide formation and monoamine oxidase inhibition. J Neurochem 95:68–78
Zheng H, Weiner LM, Bar-Am O, Epsztejn S, Cabantchik ZI, Warshawsky A, Youdim MB, Fridkin M (2005b) Design, synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer’s, Parkinson’s, and other neurodegenerative diseases. Bioorg Med Chem 13:773–783
Zheng H, Gal S, Weiner LM, Bar-Am O, Warshawsky A, Fridkin M, Youdim MB (2005c) Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases: in vitro studies on antioxidant activity, prevention of lipid peroxide formation and monoamine oxidase inhibition. J Neurochem 95(1):68–78
Zheng H, Amit T, Bar-Am O, Fridkin M, Youdim MB, Mandel SA (2012) From anti-Parkinson’s drug rasagiline to novel multitarget iron chelators with acetylcholinesterase and monoamine oxidase inhibitory and neuroprotective properties for Alzheimer’s disease. J Alzheimers Dis 30(1):1–16
Zhu W, Xie W, Pan T, Xu P, Fridkin M, Zheng H, Jankovic J, Youdim MB, Le W (2007) Prevention and restoration of lactacystin-induced nigrostriatal dopamine neuron degeneration by novel brain-permeable iron chelators. FASEB J 21(14):3835–3846
Ziegler C, Richter J, Mahr M, Gajewska A, Schiele MA, Gehrmann A, Schmidt B, Lesch KP, Lang T, Helbig-Lang S, Pauli P, Kircher T, Reif A, Rief W, Vossbeck-Elsebusch AN, Arolt V, Wittchen HU, Hamm AO, Deckert J, Domschke K (2016) MAOA gene hypomethylation in panic disorder-reversibility of an epigenetic risk pattern by psychotherapy. Transl Psychiatry 6:e773
Ziegler C, Wolf C, Schiele MA, Feric Bojic E, Kucukalic S, Sabic Dzananovic E, Goci Uka A, Hoxha B, Haxhibeqiri V, Haxhibeqiri S, Kravic N, Muminovic Umihanic M, Cima Franc A, Jaksic N, Babic R, Pavlovic M, Warrings B, Bravo Mehmedbasic A, Rudan D, Aukst-Margetic B, Kucukalic A, Marjanovic D, Babic D, Bozina N, Jakovljevic M, Sinanovic O, Avdibegovic E, Agani F, Dzubur-Kulenovic A, Deckert J, Domschke K (2018) Monoamine oxidase A gene methylation and its role in posttraumatic stress disorder: first evidence from the South Eastern Europe (SEE)-PTSD study. Int J Neuropsychopharmacol 21(5):423–432. https://doi.org/10.1093/ijnp/pyx111
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Youdim, M.B.H. Monoamine oxidase inhibitors, and iron chelators in depressive illness and neurodegenerative diseases. J Neural Transm 125, 1719–1733 (2018). https://doi.org/10.1007/s00702-018-1942-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-018-1942-9