Abstract
Background
In childhood hydrocephalus, both the amount of cerebrospinal fluid and the brain volume are relevant for the prognosis of the development and for therapy monitoring. Since classical planar measurements of ventricular size are subject to strong limitations, imprecise and neglect brain volume, 3D volumetry is most desirable. We used and evaluated the robust segmentation algorithms of the freely available FSL-toolbox in paediatric hydrocephalus patients before and after specific therapy.
Methods
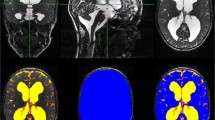
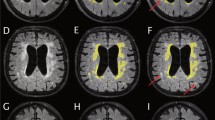
Retrospectively 76 pre- and postoperative high-resolution T2-weighted MRI sequences (true FISP, 1 mm isovoxel) were analyzed in 38 patients with paediatric hydrocephalus (mean 4.4 ± 5.1 years) who underwent surgical treatment (ventriculo-peritoneal (VP) shunt n = 22, endoscopic third ventriculostomy (ETV) n = 16). After preprocessing, the 3D-datasets were skull stripped to estimate the inner skull surface. Following, a 2 class segmentation into different tissue types (brain matter and CSF) was performed. The volumes of CSF and brain were calculated.
Results
The method could be implemented in an automated fashion in all 76 MRIs. In the VP shunt cohort, the amount of CSF (p < 0.001) decreased. Consecutively brain volume increased significantly (p < 0.001). Following ETV, CSF volume (p = 0.019) decreased significantly (p = 0.012) although the reduction was less pronounced than after shunt implantation. Brain volume expanded (p = 0.02).
Conclusion
A reliable automated segmentation of CSF and brain could be performed with the implemented algorithm. The method was able to track changes after therapy and detected significant differences in CSF and brain volumes after shunting and after ETV.




Similar content being viewed by others
Abbreviations
- CSF:
-
Cerebrospinal fluid
- VP shunt:
-
Ventriculo-peritoneal shunt
- ETV:
-
Endoscopic third ventriculostomy
- MRI:
-
Magnet resonance imaging
- VBrain:
-
Brain volume
- VCSF:
-
CSF volume
- ICV:
-
Intracranial volume
- FSL:
-
Functional Magnetic Resonance Imaging of the Brain Software Library
- BET:
-
Brain extraction tool
- FAST:
-
FMRIB’s Automated Segmentation Tool
- true FISP:
-
True fast imaging with steady-state precession
- SD:
-
Standard deviation
- FOHR:
-
Frontal occipital horn ratio
References
Ciofolo C, Barillot C (2009) Atlas-based segmentation of 3D cerebral structures with competitive level sets and fuzzy control. Med Image Anal 13:456–470. https://doi.org/10.1016/j.media.2009.02.008
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I Segmentation and surface reconstruction. Neuroimage 9:179–194. https://doi.org/10.1006/nimg.1998.0395
Del Bigio MR (2010) Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev 16:16–22. https://doi.org/10.1002/ddrr.94
Eskandari R, Abdullah O, Mason C, Lloyd KE, Oeschle AN, McAllister JP 2nd (2014) Differential vulnerability of white matter structures to experimental infantile hydrocephalus detected by diffusion tensor imaging. Childs Nerv Syst 30:1651–1661. https://doi.org/10.1007/s00381-014-2500-x
Han M, Quon J, Kim L, Shpanskaya K, Lee E, Kestle J, Lober R, Taylor M, Ramaswamy V, Edwards M, Yeom K (2019) One hundred years of innovation: automatic detection of brain ventricular volume using deep learning in a large-scale multi-institutional study (P5.6-022). Neurology 92:P5.6–P022
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl. Neuroimage 62:782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Khoo HM, Kishima H, Tani N, Oshino S, Maruo T, Hosomi K, Yanagisawa T, Kazui H, Watanabe Y, Shimokawa T, Aso T, Kawaguchi A, Yamashita F, Saitoh Y, Yoshimine T (2016) Default mode network connectivity in patients with idiopathic normal pressure hydrocephalus. J Neurosurg 124:350–358. https://doi.org/10.3171/2015.1.JNS141633
Limbrick DD Jr, Baird LC, Klimo P Jr, Riva-Cambrin J, Flannery AM, Pediatric Hydrocephalus Systematic R, Evidence-Based Guidelines Task F (2014) Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 4: cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. J Neurosurg Pediatr 14(Suppl 1):30–34. https://doi.org/10.3171/2014.7.PEDS14324
Mandell JG, Kulkarni AV, Warf BC, Schiff SJ (2015) Volumetric brain analysis in neurosurgery: part 2. Brain and CSF volumes discriminate neurocognitive outcomes in hydrocephalus. J Neurosurg Pediatr 15:125–132. https://doi.org/10.3171/2014.9.PEDS12427
Mandell JG, Langelaan JW, Webb AG, Schiff SJ (2015) Volumetric brain analysis in neurosurgery: part 1. Particle filter segmentation of brain and cerebrospinal fluid growth dynamics from MRI and CT images. J Neurosurg Pediatr 15:113–124. https://doi.org/10.3171/2014.9.PEDS12426
Mangano FT, Altaye M, McKinstry RC, Shimony JS, Powell SK, Phillips JM, Barnard H, Limbrick DD Jr, Holland SK, Jones BV, Dodd J, Simpson S, Mercer D, Rajagopal A, Bidwell S, Yuan W (2016) Diffusion tensor imaging study of pediatric patients with congenital hydrocephalus: 1-year postsurgical outcomes. J Neurosurg Pediatr 18:306–319. https://doi.org/10.3171/2016.2.PEDS15628
Mendrik AM, Vincken KL, Kuijf HJ, Breeuwer M, Bouvy WH, de Bresser J, Alansary A, de Bruijne M, Carass A, El-Baz A, Jog A, Katyal R, Khan AR, van der Lijn F, Mahmood Q, Mukherjee R, van Opbroek A, Paneri S, Pereira S, Persson M, Rajchl M, Sarikaya D, Smedby O, Silva CA, Vrooman HA, Vyas S, Wang C, Zhao L, Biessels GJ, Viergever MA (2015) MRBrainS challenge: online evaluation framework for brain image segmentation in 3T MRI scans. Comput Intell Neurosci 2015:813696. https://doi.org/10.1155/2015/813696
Moore DW, Kovanlikaya I, Heier LA, Raj A, Huang C, Chu KW, Relkin NR (2012) A pilot study of quantitative MRI measurements of ventricular volume and cortical atrophy for the differential diagnosis of normal pressure hydrocephalus. Neurol Res Int 2012:718150. https://doi.org/10.1155/2012/718150
Ogata Y, Ozaki A, Ota M, Oka Y, Nishida N, Tabu H, Sato N, Hanakawa T (2017) Interhemispheric resting-state functional connectivity predicts severity of idiopathic normal pressure hydrocephalus. Front Neurosci 11:470. https://doi.org/10.3389/fnins.2017.00470
Ragan DK, Cerqua J, Nash T, McKinstry RC, Shimony JS, Jones BV, Mangano FT, Holland SK, Yuan W, Limbrick DD Jr (2015) The accuracy of linear indices of ventricular volume in pediatric hydrocephalus: technical note. J Neurosurg Pediatr 15:547–551. https://doi.org/10.3171/2014.10.PEDS14209
Roder C, Charyasz-Leks E, Breitkopf M, Decker K, Ernemann U, Klose U, Tatagiba M, Bisdas S (2016) Resting-state functional MRI in an intraoperative MRI setting: proof of feasibility and correlation to clinical outcome of patients. J Neurosurg 125:401–409. https://doi.org/10.3171/2015.7.JNS15617
Roland JL, Griffin N, Hacker CD, Vellimana AK, Akbari SH, Shimony JS, Smyth MD, Leuthardt EC, Limbrick DD (2017) Resting-state functional magnetic resonance imaging for surgical planning in pediatric patients: a preliminary experience. J Neurosurg Pediatr 20:583–590. https://doi.org/10.3171/2017.6.PEDS1711
Schmitz B, Hagen T, Reith W (2003) Three-dimensional true FISP for high-resolution imaging of the whole brain. Eur Radiol 13:1577–1582. https://doi.org/10.1007/s00330-003-1846-3
Siasios I, Kapsalaki EZ, Fountas KN, Fotiadou A, Dorsch A, Vakharia K, Pollina J, Dimopoulos V (2016) The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review. Neurosurg Focus 41:E12. https://doi.org/10.3171/2016.6.FOCUS16192
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. https://doi.org/10.1002/hbm.10062
Toma AK, Holl E, Kitchen ND, Watkins LD (2011) Evans’ index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery 68:939–944. https://doi.org/10.1227/NEU.0b013e318208f5e0
Venkataramana NK, Mukundan CR (2011) Evaluation of functional outcomes in congenital hydrocephalus. J Pediatr Neurosci 6:4–12. https://doi.org/10.4103/1817-1745.84399
Vrooman HA, Cocosco CA, van der Lijn F, Stokking R, Ikram MA, Vernooij MW, Breteler MM, Niessen WJ (2007) Multi-spectral brain tissue segmentation using automatically trained k-Nearest-Neighbor classification. Neuroimage 37:71–81. https://doi.org/10.1016/j.neuroimage.2007.05.018
Warf B, Ondoma S, Kulkarni A, Donnelly R, Ampeire M, Akona J, Kabachelor CR, Mulondo R, Nsubuga BK (2009) Neurocognitive outcome and ventricular volume in children with myelomeningocele treated for hydrocephalus in Uganda. J Neurosurg Pediatr 4:564–570. https://doi.org/10.3171/2009.7.PEDS09136
Yepes-Calderon F, Nelson MD, McComb JG (2018) Automatically measuring brain ventricular volume within PACS using artificial intelligence. PLoS One 13:e0193152. https://doi.org/10.1371/journal.pone.0193152
Yuan W, McKinstry RC, Shimony JS, Altaye M, Powell SK, Phillips JM, Limbrick DD Jr, Holland SK, Jones BV, Rajagopal A, Simpson S, Mercer D, Mangano FT (2013) Diffusion tensor imaging properties and neurobehavioral outcomes in children with hydrocephalus. AJNR Am J Neuroradiol 34:439–445. https://doi.org/10.3174/ajnr.A3218
Yuan W, Harpster K, Jones BV, Shimony JS, McKinstry RC, Weckherlin N, Powell SS, Barnard H, Engsberg J, Kadis DS, Dodd J, Altaye M, Limbrick DD, Holland SK, Simpson SM, Bidwell S, Mangano FT (2016) Changes of white matter diffusion anisotropy in response to a 6-week iPad application-based occupational therapy intervention in children with surgically treated hydrocephalus: a pilot study. Neuropediatrics 47:336–340. https://doi.org/10.1055/s-0036-1584938
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20:45–57. https://doi.org/10.1109/42.906424
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (University Hospital Tübingen, Germany) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Additional information
Comments
In order to assess CSF spaces in hydrocephalic patients, modern imaging techniques may add significant information, e.g. in follow up after treatment, but also in respect of investigating functional outcome correlated with changes of brain structures and CSF spaces. The authors are presenting one example of these innovative techniques.
Angela-Martina Messing-Junger
Bonn, Germany
The authors review a population of children with hydrocephalus (n = 76) and use segmentation algorithms obtained from freely available online software to measure brain and ventricular volume before and after insertion of a ventriculoperitoneal shunt (n = 22) or endoscopic third ventriculostomy (n = 16). They conclude that the software confirmed a reduction in ventricular size and an increase in brain volume, and argue that this technique is potentially more objective than measurement of traditional parameters of ventricular size, such as Evans ratio or frontal occipital horn ratio, in the diagnosis of hydrocephalus and its response to treatment. The authors describe their methods well, and explain clearly which MRI sequences they have used. They discuss the benefits of the software they have chosen; it is free, easily available and, unlike many others, appears less labour intensive. They argue that accurate ventricular and brain volume measurement is an important pre-requisite to a deeper understanding of the correlation between ventricular and brain size with cognitive development. This is an important study, and, as neurosurgery and neuroradiology progress towards more accurate and more automated evaluation of ventricular and brain volume, it becomes particularly relevant.
Kristian Aquilina
London, UK
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Neurosurgery
Rights and permissions
About this article
Cite this article
Grimm, F., Edl, F., Gugel, I. et al. Automatic volumetry of cerebrospinal fluid and brain volume in severe paediatric hydrocephalus, implementation and clinical course after intervention. Acta Neurochir 162, 23–30 (2020). https://doi.org/10.1007/s00701-019-04143-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-04143-5