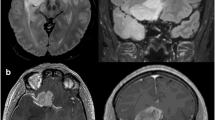
Abstract
Background
High-field intraoperative MRI (IoMRI) is part of the neurosurgical armamentarium to improve the extent of glioma resection (EOR).
Objective
To report our oncological and functional outcomes using IoMRI for neuronavigated glioma surgery.
Methods
In this prospective monocentric study, we reported 100 consecutive adult patients operated on for glioma using IoMRI with neuronavigation, under general anesthesia without intraoperative stimulation, from July 2014 to April 2017. The volumetric evaluation was based on the FLAIR hypersignal for non-enhancing tumors after Gadolinium infusion and on the T1 hypersignal after Gadolinium infusion for enhancing tumors. We evaluated the surgical workflow, the EOR and the clinical outcomes (using Karnofsky performance score (KPS)).
Results
Sixty-nine patients underwent one IoMRI, and 31 from two IoMRI controls. At first IoMRI, the median tumor residue was higher in the FLAIR group than in the T1G+ group whereas no more difference was reported after the second IoMRI between the 2 groups (p = 0.56). Additional resection was performed 6 times more frequently in the FLAIR group (OR = 5.7, CI (1.9–17)). The median EOR was 100% (IQR, 93.6–100) whatever the tumor type (first surgery or recurrence) and location. Higher residues were reported only in the insula area (p = 0.004). The median KPS was 90 (IQR, 80–100) at discharge, 3, 6 and 12 months after surgery, with no statistical difference between low- and high-grade gliomas (p = 0.34).
Conclusion
IoMRI neuronavigated surgery provided maximal EOR whatever the type of glioma and location. IoMRI was all the more useful for non- or minimally enhancing tumors. The step by step surgical resection, introducing the concept of “staged volume” surgery, ensured a high security for the surgeon and low permanent morbidity for the patients.





Similar content being viewed by others
References
Barone DG, Lawrie TA, Hart MG (2014) Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev 1:CD009685. https://doi.org/10.1002/14651858.CD009685.pub2
Brown PD, Maurer MJ, Rummans TA, Pollock BE, Ballman KV, Sloan JA, Boeve BF, Arusell RM, Clark MM, Buckner JC (2005) A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery 57:495
Brzozowska A, Toruń A, Mazurkiewicz M (2015) The impact of surgery on the efficacy of adjuvant therapy in glioblastoma multiforme. Adv Clin Exp Med 24:279–287. https://doi.org/10.17219/acem/40456
Coburger J, Merkel A, Scherer M, Schwartz F, Gessler F, Roder C, Pala A, Konig R, Bullinger L, Nagel G, Jungk C, Bisdas S, Nabavi A, Ganslandt O, Seifert V, Tatagiba M, Senft C, Mehdorn M, Unterberg AW, Rossler K, Wirtz CR (2016) Low-grade glioma surgery in intraoperative magnetic resonance imaging: results of a multicenter retrospective assessment of the German study group for intraoperative magnetic resonance imaging. Neurosurgery 78:775–786. https://doi.org/10.1227/NEU.0000000000001081
D'Andrea G, Familiari P, Di Lauro A, Angelini A, Sessa G (2016) Safe resection of gliomas of the dominant angular gyrus availing of preoperative FMRI and intraoperative DTI: preliminary series and surgical technique. World Neurosurg 87:627–639. https://doi.org/10.1016/j.wneu.2015.10.076
Della Puppa A, De Pellegrin S, d'Avella E, Gioffre G, Rossetto M, Gerardi A, Lombardi G, Manara R, Munari M, Saladini M, Scienza R (2013) 5-aminolevulinic acid (5-ALA) fluorescence guided surgery of high-grade gliomas in eloquent areas assisted by functional mapping. Our experience and review of the literature. Acta Neurochir 155:965–972; discussion 972. https://doi.org/10.1007/s00701-013-1660-x
Dupont C, Vermandel M, Leroy HA, Quidet M, Lecomte F, Delhem N, Mordon S, Reyns N (2018) INtraoperative photoDYnamic therapy for GliOblastomas: study protocol for a phase I clinical trial. Neurosurgery. https://doi.org/10.1093/neuros/nyy324
Ehrle N, Henry A, Pesa A, Bakchine S (2011) Assessment of sociocognitive functions in neurological patients presentation of a French adaptation of two tools and implementation in frontal dementia. Geriatr Psychol Neuropsychiatr Vieil 9:117–128. https://doi.org/10.1684/pnv.2010.0252
Eseonu CI, Rincon-Torroella J, ReFaey K, Lee YM, Nangiana J, Vivas-Buitrago T, Quinones-Hinojosa A (2017) Awake craniotomy vs craniotomy under general anesthesia for perirolandic gliomas: evaluating perioperative complications and extent of resection. Neurosurgery 81:481–489. https://doi.org/10.1093/neuros/nyx023
Habets EJ, Kloet A, Walchenbach R, Vecht CJ, Klein M, Taphoorn MJ (2014) Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochir 156:1451–1459. https://doi.org/10.1007/s00701-014-2115-8
Hervey-Jumper SL, Li J, Osorio JA, Lau D, Molinaro AM, Benet A, Berger MS (2016) Surgical assessment of the insula. Part 2: validation of the Berger-Sanai zone classification system for predicting extent of glioma resection. J Neurosurg 124:482–488. https://doi.org/10.3171/2015.4.JNS1521
Ius T, Isola M, Budai R, Pauletto G, Tomasino B, Fadiga L, Skrap M (2012) Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg 117:1039–1052. https://doi.org/10.3171/2012.8.JNS12393
Kuhnt D, Ganslandt O, Schlaffer SM, Buchfelder M, Nimsky C (2011) Quantification of glioma removal by intraoperative high-field magnetic resonance imaging: an update. Neurosurgery 69:852–862; discussion 862-853. https://doi.org/10.1227/NEU.0b013e318225ea6b
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. https://doi.org/10.3171/jns.2001.95.2.0190
Leroy HA, Delmaire C, Le Rhun E, Drumez E, Lejeune JP, Reyns N (2018) High-field intraoperative MRI in glioma surgery: a prospective study with volumetric analysis of extent of resection and functional outcome. Neurochirurgie 64:155–160. https://doi.org/10.1016/j.neuchi.2018.02.003
Maesawa S, Fujii M, Nakahara N, Watanabe T, Wakabayashi T, Yoshida J (2010) Intraoperative tractography and motor evoked potential (MEP) monitoring in surgery for gliomas around the corticospinal tract. World Neurosurg 74:153–161. https://doi.org/10.1016/j.wneu.2010.03.022
Maldaun MV, Khawja SN, Levine NB, Rao G, Lang FF, Weinberg JS, Tummala S, Cowles CE, Ferson D, Nguyen AT, Sawaya R, Suki D, Prabhu SS (2014) Awake craniotomy for gliomas in a high-field intraoperative magnetic resonance imaging suite: analysis of 42 cases. J Neurosurg 121:810–817. https://doi.org/10.3171/2014.6.JNS132285
McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, AR Q√±o-H (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110:156–162. https://doi.org/10.3171/2008.4.17536
Mohammadi AM, Sullivan TB, Barnett GH, Recinos V, Angelov L, Kamian K, Vogelbaum MA (2014) Use of high-field intraoperative magnetic resonance imaging to enhance the extent of resection of enhancing and nonenhancing gliomas. Neurosurgery 74:339. https://doi.org/10.1227/NEU.0000000000000278
Oppenlander ME, Wolf AB, Snyder LA, Bina R, Wilson JR, Coons SW, Ashby LS, Brachman D, Nakaji P, Porter RW, Smith KA, Spetzler RF, Sanai N (2014) An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg 120:846–853. https://doi.org/10.3171/2013.12.JNS13184
Ottenhausen M, Krieg SM, Meyer B, Ringel F (2015) Functional preoperative and intraoperative mapping and monitoring: increasing safety and efficacy in glioma surgery. Neurosurg Focus 38:E3. https://doi.org/10.3171/2014.10.FOCUS14611
Quick J, Gessler F, Dutzmann S, Hattingen E, Harter PN, Weise LM, Franz K, Seifert V, Senft C (2014) Benefit of tumor resection for recurrent glioblastoma. J Neuro-Oncol 117:365–372. https://doi.org/10.1007/s11060-014-1397-2
Reyns N, Leroy HA, Delmaire C, Derre B, Le-Rhun E, Lejeune JP (2017) Intraoperative MRI for the management of brain lesions adjacent to eloquent areas. Neurochirurgie 63:181–188. https://doi.org/10.1016/j.neuchi.2016.12.006
Sanai N, Polley M-YY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. https://doi.org/10.3171/2011.2.JNS10998
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12:997–1003. https://doi.org/10.1016/s1470-2045(11)70196-6
Suero Molina E, Schipmann S, Stummer W (2017) Maximizing safe resections: the roles of 5-aminolevulinic acid and intraoperative MR imaging in glioma surgery-review of the literature. Neurosurg Rev. https://doi.org/10.1007/s10143-017-0907-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Comité d’éthique institutionnel du CHU de Lille) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Tumor - Glioma
Rights and permissions
About this article
Cite this article
Leroy, HA., Delmaire, C., Le Rhun, E. et al. High-field intraoperative MRI and glioma surgery: results after the first 100 consecutive patients. Acta Neurochir 161, 1467–1474 (2019). https://doi.org/10.1007/s00701-019-03920-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-03920-6