Abstract
Background
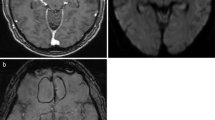
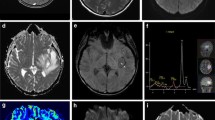
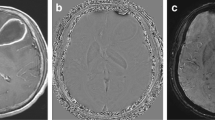
Diffusion-weighted magnetic resonance imaging (MRI-DWI) is the modality of choice for detecting intracranial abscesses; however, it is unclear whether prior brain surgery has an influence on its diagnostic value. Thus, we assessed the robustness of MRI-DWI and determination of an ADC cutoff value for detecting intracranial abscesses in patients who underwent brain surgery.
Methods
We retrospectively evaluated 19 patients prior to surgery for postoperative supratentorial parenchymal abscesses by means of MRI-DWI. Forty randomly selected patients with routine postoperative MRI-DWI were used for comparative analyses. Clinical and serum biomarkers (C-reactive protein, interleukin-6, white blood cell count) as well as from results of early postoperative imaging findings (computed tomography and/or MRI scan) were recorded. Additionally, ADC values, T1±gadolinium, and T2/fluid-attenuated inversion recovery sequences were investigated.
Results
After initial surgery, early postoperative control imaging showed evidence of hemorrhage and/or hemostatic agents within the resection cavity in 10/19 patients of the abscess group and in 16/40 patients of the control group. No postoperative ischemia was detected. Neither hemostatic agents nor blood affected the mean ADC values in both the reference group (blood 2.96 ± 0.22 × 10−3 mm2/s vs. no blood 2.95 ± 0.26 × 10−3 mm2/s, p = 0.076) and in the abscess group (blood 0.87 ± 0.07 × 10−3 mm2/s vs. no blood 0.76 ± 0.06 × 10−3 mm2/s, p = 0.128). The mean ADC value within the resection cavity was significantly lower in the abscess group (1.5 T 0.88 ± 0.41 vs. 2.88 ± 0.20 × 10−3 mm2/s, p < .01; 3.0 T 0.75 ± 0.24 vs. 3.02 ± 0.26 × 10−3 mm2/s, p < 0.01). The optimal ADC cut-off for the differentiation of an abscess from normal postoperative findings was found at 1.87 × 10−3 mm2/s (area-under-the-curve 1.0, sensitivity = 100%, specificity = 100%). Moreover, no differences between the abscess patients and the control group were seen with respect to the analyzed serum biomarkers.
Conclusion
MRI-DWI provides a robust tool to discriminate postoperative abscess formation from normal postoperative changes.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CT:
-
Computed tomography
- CRP:
-
C-reactive protein
- CSF:
-
Cerebrospinal fluid
- DWI:
-
Diffusion-weighted imaging
- IL-6:
-
Interleukin-6
- MRI:
-
Magnetic resonance imaging
- WBCC:
-
White blood cell count
References
Anthofer J, Wester M, Zeman F, Brawanski A, Schebesch KM (2016) Case-control study of patients at risk of medical complications after elective craniotomy. World Neurosurg 91:58–65
Asadullah K, Woiciechowsky C, Döcke WD, Egerer K, Kox WJ, Vogel S, Sterry W, Volk HD (1995) Very low monocytic HLA-DR expression indicates high risk of infection--immunomonitoring for patients after neurosurgery and patients during high dose steroid therapy. Eur J Emerg Med 2:184–190
Belli G, Busoni S, Ciccarone A, Coniglio A, Esposito M, Giannelli M, Mazzoni LN, Nocetti L, Sghedoni R, Tarducci R, Zatelli G, Anoja RA, Belmonte G, Bertolino N, Betti M, Biagini C, Ciarmatori A, Cretti F, Fabbri E, Fedeli L, Filice S, Fulcheri CP, Gasperi C, Mangili PA, Mazzocchi S, Meliadò G, Morzenti S, Noferini L, Oberhofer N, Orsingher L, Paruccini N, Princigalli G, Quattrocchi M, Rinaldi A, Scelfo D, Freixas GV, Tenori L, Zucca I, Luchinat C, Gori C, Gobbi G, Italian Association of Physics in Medicine (AIFM) Working Group on MR Intercomparison (2016) Quality assurance multicenter comparison of different MR scanners for quantitative diffusion-weighted imaging. J Magn Reson Imaging 43:213–219
Berndt M, Lange N, Ryang YM, Meyer B, Zimmer C, Hapfelmeier A, Wantia N, Gempt J, Lummel N (2018) Value of diffusion-weighted imaging in the diagnosis of postoperative intracranial infections. World Neurosurg 118:e245–e253
Brouwer MC, Coutinho JM, van de Beek D (2014a) Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology 82:806–813
Brouwer MC, Tunkel AR, McKhann GM 2nd, van de Beek D (2014b) Brain abscess. N Engl J Med 371:447–456
Bükte Y, Paksoy Y, Genç E, Uca AU (2005) Role of diffusion-weighted MR in differential diagnosis of intracranial cystic lesions. Clin Radiol 60:375–383
Castillo M, Mukherji SK (2000) Diffusion-weighted imaging in the evaluation of intracranial lesions. Semin Ultrasound CT MR 21:405–416
Chang SC, Lai PH, Chen WL, Weng HH, Ho JT, Wang JS, Chang CY, Pan HB, Yang CF (2002) Diffusion-weighted MRI features of brain abscess and cystic or necrotic brain tumors: comparison with conventional MRI. Clin Imaging 26:227–236
Ebisu T, Tanaka C, Umeda M, Kitamura M, Naruse S, Higuchi T, Ueda S, Sato H (1996) Discrimination of brain abscess from necrotic or cystic tumors by diffusion-weighted echo planar imaging. Magn Reson Imaging 14:1113–1116
Farrell CJ, Hoh BL, Pisculli ML, Henson JW, Barker FG 2nd, Curry WT Jr (2008) Limitations of diffusion-weighted imaging in the diagnosis of postoperative infections. Neurosurgery 62:577–583
Golebiowski A, Drewes C, Gulati S, Jakola AS, Solheim O (2015) Is duration of surgery a risk factor for extracranial complications and surgical site infections after intracranial tumor operations? Acta Neurochir 157:235–240
Grech-Sollars M, Hales PW, Miyazaki K, Raschke F, Rodriguez D, Wilson M, Gill SK, Banks T, Saunders DE, Clayden JD, Gwilliam MN, Barrick TR, Morgan PS, Davies NP, Rossiter J, Auer DP, Grundy R, Leach MO, Howe FA, Peet AC, Clark CA (2015) Multi-centre reproducibility of diffusion MRI parameters for clinical sequences in the brain. NMR Biomed 28:468–485
Grimstad IA, Hirschberg H, Rootwelt K (1992) 99mTc-hexamethylpropyleneamine oxime leukocyte scintigraphy and C-reactive protein levels in the differential diagnosis of brain abscesses. J Neurosurg 77:732–736
Kang BK, Na DG, Ryoo JW, Byun HS, Roh HG, Pyeun YS (2001) Diffusion-weighted MR imaging of intracerebral hemorrhage. Korean J Radiol 2:183–191
Kim YJ, Moon KS, Kim SK, Kang SJ, Lee KH, Jang WY, Jung TY, Kim IY, Jung S (2014) The difference in diffusion-weighted imaging with apparent diffusion coefficient between spontaneous and postoperative intracranial infection. Br J Neurosurg 28:765–770
Korinek AM (1997) Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. The French Study Group of Neurosurgical Infections, the SEHP, and the C-CLIN Paris-Nord. Service Epidémiologie Hygiène et Prévention. Neurosurgery 41:1073–1079
Kourbeti IS, Vakis AF, Ziakas P, Karabetsos D, Potolidis E, Christou S, Samonis G (2015) Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg 122:1113–1119
Lai PH, Ho JT, Chen WL, Hsu SS, Wang JS, Pan HB, Yang CF (2002) Brain abscess and necrotic brain tumor: discrimination with proton MR spectroscopy and diffusion-weighted imaging. AJNR Am J Neuroradiol 23:1369–1377
Levy RM (1994) Brain abscess and subdural empyema. Curr Opin Neurol 7:223–228
Leuthardt EC, Wippold FJ 2nd, Oswood MC, Rich KM (2002) Diffusion-weighted MR imaging in the preoperative assessment of brain abscesses. Surg Neurol 58:395–402
Lotan E, Hoffmann C, Fardman A, Ziv-Baran T, Komisar O, Harnof S (2016) Postoperative versus spontaneous intracranial abscess: diagnostic value of the apparent diffusion coefficient for accurate assessment. Radiology 281:168–174
Nadal Desbarats L, Herlidou S, de Marco G, Gondry-Jouet C, Le Gars D, Deramond H, Idy-Peretti I (2003) Differential MRI diagnosis between brain abscesses and necrotic or cystic brain tumors using the apparent diffusion coefficient and normalized diffusion-weighted images. Magn Reson Imaging 21:645–650
Neidert MC, Karlin K, Actor B, Regli L, Bozinov O, Burkhardt JK (2015) Preoperative C-reactive protein predicts the need for repeated intracerebral brain abscess drainage. Clin Neurol Neurosurg 131:26–30
Ogura A, Tamura T, Ozaki M, Doi T, Fujimoto K, Miyati T, Ito Y, Maeda F, Tarewaki H, Takahashi M Apparent diffusion coefficient value is not dependent on magnetic resonance systems and field strength under fixed imaging parameters in brain. J Comput Assist Tomogr 39:760–765
Rana S, Albayram S, Lin DD, Yousem DM (2002) Diffusion-weighted imaging and apparent diffusion coefficient maps in a case of intracerebral abscess with ventricular extension. AJNR Am J Neuroradiol 23:109–112
Sener RN (2001) Diffusion MRI: apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput Med Imaging Graph 25:299–326
Toh CH, Wei KC, Ng SH, Wan YL, Lin CP, Castillo M (2011) Differentiation of brain abscesses from necrotic glioblastomas and cystic metastatic brain tumors with diffusion tensor imaging. AJNR Am J Neuroradiol 32:1646–1651
Tomar V, Yadav A, Rathore RK, Verma S, Awasthi R, Bharadwaj V, Ojha BK, Prasad KN, Gupta RK (2011) Apparent diffusion coefficient with higher b-value correlates better with viable cell count quantified from the cavity of brain abscess. AJNR Am J Neuroradiol 32:2120–2125
Valentini LG, Casali C, Chatenoud L, Chiaffarino F, Uberti-Foppa C, Broggi G (2008) Surgical site infections after elective neurosurgery: a survey of 1747 patients. Neurosurgery 62:88–95
Wendl CM, Müller S, Eiglsperger J, Fellner C, Jung EM, Meier JK (2015) Diffusion-weighted imaging in oral squamous cell carcinoma using 3 tesla MRI: is there a chance for preoperative discrimination between benign and malignant lymph nodes in daily clinical routine? Acta Radiol 57:939–946
Yu Y, Li HJ (2017) Diagnostic and prognostic value of procalcitonin for early intracranial infection after craniotomy. Braz J Med Biol Res 50:e6021
Zhan R, Zhu Y, Shen Y, Shen J, Tong Y, Yu H, Wen L (2014) Post-operative central nervous system infections after cranial surgery in China: incidence, causative agents, and risk factors in 1,470 patients. Eur J Clin Microbiol Infect Dis 33:861–866
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (ethics committee of the Ludwig-Maximilians-University Munich, Germany) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Infection
Rights and permissions
About this article
Cite this article
Schwartz, C., Lenski, M., Romagna, A. et al. Diffusion-weighted magnetic resonance imaging for detection of postoperative intracranial pyogenic abscesses in neurosurgery. Acta Neurochir 161, 985–993 (2019). https://doi.org/10.1007/s00701-019-03875-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-03875-8