Abstract
Background
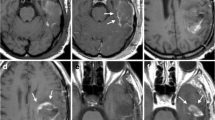
This study aimed to investigate the incidence of residual tumour after resection of brain metastases using early postoperative magnetic resonance imaging (MRI) and the influence of residual tumour on overall patient survival.
Methods
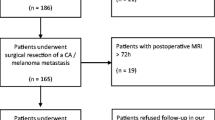
Data from 72 consecutive adult patients undergoing surgery for cerebral metastases over an 18-month study period were retrospectively collected. Early postoperative MRI was used to determine the presence of postoperative residual tumour. Patients were divided into three groups according to the presence of tumour remnant on early postoperative MRI: “no residual tumour”, “non-measurable residual tumour” and “measurable residual tumour”. Survival analysis (mean estimate survival time) was performed using the Kaplan–Meier and log-rank (mantel cox) tests and compared between groups. Surgical reports were evaluated with regard to the surgeon statement about intraoperative extent of resection (EOR) and compared with the presence of tumour remnant found on the early postoperative MRI.
Results
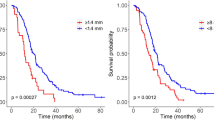
Sixty-eight procedures were followed by early postoperative MRI. MRI verified the presence of “measurable residual tumour” following 15 procedures (22%). MRI confirmed complete resection in 57%. Gross total resection was described by the operating surgeon in 85% of the procedures. There was a significant difference in survival time after surgery between the group having no residual tumour on MRI and the group with measurable residual tumour (p = 0.025). This difference could not be explained by the differences in postoperative radiation therapy. The longest survival was found in patients with non-measurable and no residual tumour on early postoperative MRI, who also received postoperative radiotherapy.
Conclusion
Residual tumour was seen on MRI after 22% of the procedures. The intraoperative assessment of EOR performed by the surgeon diverged from the early postoperative MRI in 40% of procedures. Correct assessment of residual tumour thus requires early postoperative MRI. Measurable residual tumour on early postoperative MRI was associated with shorter overall survival independent on postoperative radiotherapy.

Similar content being viewed by others
Abbreviations
- BrM:
-
Brain metastases
- MRI:
-
Magnetic resonance imaging
- EPMRI:
-
Early postoperative magnetic resonance imaging
- EOR:
-
Extent of resection
- GTR:
-
Gross total resection
- OUH:
-
Odense University Hospital
- MG:
-
Malignant glioma
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- WBRT:
-
Whole brain radiation therapy
- SRS:
-
Stereotactic radiosurgery
- KPS:
-
Karnofsky performance score
References
Ahn J, Lee S, Kim S et al (2012) Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg JNS 116(5):984–993 https://thejns.org/view/journals/j-neurosurg/116/5/article-p984.xml. Accessed Nov 2018
Albert FK, Forsting M, Sartor K et al (1994) Early postoperative magnetic resonance imaging after resection of malignant glioma; objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34:45–61
Al-Shamy G, Sawaya R (2009) Management of brain metastases: the indispensable role of surgery. J Neurooncol 92:275–282. https://doi.org/10.1007/s11060-009-9839-y
Arbejdsgruppen for NKR, Hjernemetastaser (Working group for Danish national clinical guidelines brain metastases) (2014) National Clinical guideline Brain metastases (sundhedsstyrelsen): http://www.dnks.dk/fileadmin/user_upload/NKR_hjernemetastaser_klinisk_retningslinje_2014.pdf. Accessed July 2016
Benveniste RJ, Ferraro N, Tsimpas A (2014) Yield and utility of routine postoperative imaging after resection of brain metastases. J Neurooncol 118:363–367. https://doi.org/10.1007/s11060-014-1440-3
Berghoff AS, Rajky O, Winkler F et al (2013) Invasion patterns in brain metastases of solid cancers. Neuro-Oncology 15:1664–1672. https://doi.org/10.1093/neuonc/not112
Danish Neuro Oncological (working) Group (2014) Clinical guidelines for treatment of gliomas: http://www.dnog.dk/assets/files/Retningslinier%20PDF/DNOG-2014-Gliom-Retningslinje.pdf. Accessed july 2016
Danish Neurosurgical Society (DNKS) (2015) Guidelines for treatment of brain metastases / Retningslinier vedrørende behandling af hjernemetastaser: http://www.dnks.dk/fileadmin/user_upload/2015/DNKS_retningslinier_hjernemet_version_21_april_20151.pdf. Accessed July 2016
Höhne J, Hohenberger C, Proescholdt M et al (2017) Fluorescein sodium-guided resection of cerebral metastases—an update. Acta Neurochir 159:363. https://doi.org/10.1007/s00701-016-3054-3
Kamp MA, Rapp M, Buhner J et al (2015) Early postoperative magnet resonance tomography after resection of cerebral metastases. Acta Neurochir 157:1573–1580. https://doi.org/10.1007/s00701-015-2479-4
Kamp MA, Rapp M, Slotty PJ et al (2015) Incidence of local in-brain progression after supramarginal resection of cerebral metastases. Acta Neurochir 157:905–911. https://doi.org/10.1007/s00701-015-24-9
Lim CSK, Grundy PL (2013) Effectiveness and outcomes of surgery for cerebral metastases. Br J Neurosurg 27:654–657. https://doi.org/10.3109/02688697.2013.771729
Lin X, DeAngelis LM (2015) Treatment of brain metastases. J Clin Oncol 33:3475–3484. https://doi.org/10.1200/JCO.2015.60.9503
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278. https://doi.org/10.1016/s1470-2045(15)70057-4]
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18(8):1040–1048
Orringer D, Lau D, Khatri S et al (2012) Extent of resection in patients with glioblastoma; limiting factors, perception of resectability and effect on survival. J Neurosurg 117(5):851–859. https://doi.org/10.3171/2012.8.JNS12234]
Preston JK, Masciopinto J, Salamat MS et al (1999) Tumour cell dispersion by the ultrasonic aspirator during brain tumour resection. Br J Neurosurg 13(5):486–489
Rogne SG, Ronning P, Helseth E et al (2012) Craniotomy for brain metastases: a consecutive series of 316 patients. Acta Neurol Scand 126(1):23–31. https://doi.org/10.1111/j.1600-0404.2011.01590.x
Schackert G, Lindner C, Petschke S et al (2013) Retrospective study of 127 surgically treated patients with multiple brain metastases: indication, prognostic factors, and outcome. Acta Neurochir 155(3):379–387. https://doi.org/10.1007/s00701-012-1606-8
Schebesch KM, Hoehne J, Hohenberger C et al (2015) Fluorescein sodium-guided resection of cerebral metastases-experience with the first 30 patients. Acta Neurochir 157(6):899–904. https://doi.org/10.1007/s00701-015-2395-7
Stummer W, Pichmeier U, Meinel T et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7(5):392–401
Yoo H, Kim YZ, Nam BH ea (2009) Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg 110(4):730–736. https://doi.org/10.3171/2008.8.JNS08448
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is a retrospective study. For this type of study, formal consent from the patients is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Brain Tumors
Rights and permissions
About this article
Cite this article
Olesrud, I.C., Schulz, M.K., Marcovic, L. et al. Early postoperative MRI after resection of brain metastases—complete tumour resection associated with prolonged survival. Acta Neurochir 161, 555–565 (2019). https://doi.org/10.1007/s00701-019-03829-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-03829-0