Abstract
Background
Cervical spondylotic myelopathy (CSM) is the most common cause of spinal cord dysfunction, potentially leading to severe disability. Abnormal cervical spine magnetic resonance imaging (MRI) and motor evoked potentials (MEPs) are independent predictors of disease progression. Abnormal MRI is accompanied by various activation changes in functional brain MRI (fMRI), whereas preoperative and postoperative fMRI adaptations associated with abnormal preoperative MEP remain unknown.
Methods
Twenty patients (9 males, average age 56.6) with evidence of spinal cord compression on MRI and clinical signs of mild CSM were included. Participants were classified according to their preoperative MEP and underwent three brain fMRI examinations: before surgery, 6, and 12 months after surgery while performing repeated extension-flexion of each wrist.
Results
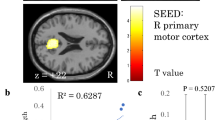
Functional MRI activation was compared between two subsets of patients, with normal and clearly abnormal MEP (right wrist: 8 vs. 8; left wrist: 7 vs. 9). At baseline, abnormal MEPs were associated with hyperactivation in the cerebellum. At the first follow-up, further hyperactivations emerged in the contralateral sensorimotor cortices and persisted for 1 year. In normal baseline MEP, activation mostly decreased in the ipsilateral sensorimotor cortex postoperatively. The ipsilateral sensorimotor activation after 1-year follow-up correlated with baseline MEP.
Conclusions
Abnormal corticospinal MEP findings in cervical spondylotic myelopathy were associated with differences in brain activation, which further increased after spinal cord decompression and did not resolve within 12-month follow-up. In summary, surgery may come too late for those patients with abnormal MEP to recover completely despite their mild clinical signs and symptoms.




Similar content being viewed by others
Abbreviations
- AP:
-
anteroposterior
- ANOVA:
-
analysis of variance
- CMCT:
-
central motor conduction time
- CSM:
-
cervical spondylotic myelopathy
- DWI:
-
diffusion-weighted imaging
- EPI:
-
echo planar imaging
- fMRI:
-
functional MR imaging
- FWHM:
-
full width at half maximum
- GLM:
-
general linear model
- Group A:
-
abnormal MEP
- Group B:
-
borderline MEP
- Group N:
-
normal MEP
- LE:
-
lower extremity
- LW:
-
left wrist
- M0:
-
month 0
- M6:
-
month 6
- M12:
-
month 12
- MEP:
-
motor evoked potentials
- mJOA:
-
modified Japanese Orthopaedic Association score
- MNI:
-
Montreal Neurological Institute
- MPRAGE:
-
magnetization prepared rapid acquisition gradient echo
- MRI:
-
magnetic resonance imaging
- NDI:
-
Neck Disability Index
- PEEK:
-
polyetheretherketon
- ROI:
-
region of interest
- RW:
-
right wrist
- SD:
-
standard deviation
- SEP:
-
somatosensory evoked potentials
- SMA:
-
supplementary motor area
- UE:
-
upper extremity
- VAS:
-
visual analogue scale
References
Aleksanderek I, Stevens TK, Goncalves S, Bartha R, Duggal N (2017) Metabolite and functional profile of patients with cervical spondylotic myelopathy. J Neurosurg Spine:1–7
Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC (2000) Functional MRI cerebral activation and deactivation during finger movement. Neurology 54(1):135–142
Bednařík J, Kadaňka Z, Dušek L, Keřkovský M, Voháňka S, Novotný O, Urbánek I, Kratochvílová D (2008) Presymptomatic spondylotic cervical myelopathy: an updated predictive model. Eur Spine J 17(3):421–431
Bednařík J, Kadaňka Z, Dušek L, Novotný O, Šurelová D, Urbánek I, Prokeš B (2004) Presymptomatic spondylotic cervical cord compression. Spine 29(20):2260–2269
Bednařík J, Kadaňka Z, Voháňka S, Stejskal L, Vlach O, Schröder R (1999) The value of somatosensory- and motor-evoked potentials in predicting and monitoring the effect of therapy in spondylotic cervical myelopathy. Prospective randomized study. Spine 24(15):1593–1598
Dong Y, Holly LT, Albistegui-Dubois R, Yan X, Marehbian J, Newton JM, Dobkin BH (2008) Compensatory cerebral adaptations before and evolving changes after surgical decompression in cervical spondylotic myelopathy. J Neurosurg Spine 9(6):538–551
Duggal N, Rabin D, Bartha R, Barry RL, Gati JS, Kowalczyk I, Fink M (2010) Brain reorganization in patients with spinal cord compression evaluated using fMRI. Neurology 74(13):1048–1054
Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL (2006) Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 9(Pt 2):58–66
Holly LT, Dong Y, Albistegui-DuBois R, Marehbian J, Dobkin B (2007) Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine 6(6):544–551
Hrabálek L, Hluštík P, Hok P, Čecháková E, Wanek T, Otruba P, Vaverka M, Kaňovský P (2015) Influence of cervical spondylotic spinal cord compression on cerebral cortical adaptation. Radiological study. Acta Chir orthop Traum čech 82(6):404–411
Hrabálek L, Hluštík P, Hok P, Wanek T, Otruba P, Čecháková E, Vaverka M, Kaňovský P (2014) Effects of spinal cord decompression in patients with cervical spondylotic myelopathy oncortical brain activations. Rozhledy v chirurgii : mesicnik Ceskoslovenske chirurgicke spolecnosti 93(11):530–535
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012) FSL. NeuroImage 62(2):782–790
Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC (2007) Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil Neural Repair 21(6):527–538
Kadaňka Z, Keřkovský M, Bednařík J, Jarkovský J (2007) Cross-sectional transverse area and hyperintensities on magnetic resonance imaging in relation to the clinical picture in cervical spondylotic myelopathy. Spine 32(23):2573–2577
Kalsi-Ryan S, Karadimas SK, Fehlings MG (2013) Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 19(4):409–421
Keřkovský M, Bednařík J, Dušek L, Šprláková-Puková A, Urbánek I, Mechl M, Válek V, Kadaňka Z (2012) Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine 37(1):48–56
Kowalczyk I, Duggal N, Bartha R (2012) Proton magnetic resonance spectroscopy of the motor cortex in cervical myelopathy. Brain 135(Pt 2):461–468
Manto M, Bower J, Conforto A et al (2012) Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum 11(2):457–487
Penhune VB, Steele CJ (2012) Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav Brain Res 226(2):579–591
Tam S, Barry RL, Bartha R, Duggal N (2010) Changes in functional magnetic resonance imaging cortical activation after decompression of cervical spondylosis: case report. Neurosurgery 67(3):E863–E864
Acknowledgements
This study was supported by the grant IGA MZ ČR NT 14609.
Funding
This study was supported by the grant IGA MZ ČR NT 14609.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Prior to study initiation, the study protocol was approved by the institutional ethics committee. Informed consent was obtained from each study participant prior to any study procedure.
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Animal experiments
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Hrabálek, L., Hok, P., Hluštík, P. et al. Longitudinal brain activation changes related to electrophysiological findings in patients with cervical spondylotic myelopathy before and after spinal cord decompression: an fMRI study. Acta Neurochir 160, 923–932 (2018). https://doi.org/10.1007/s00701-018-3520-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-018-3520-1