Abstract
Introduction
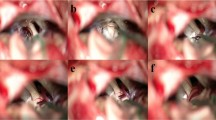
Vascular compression is the main pathogenetic factor in apparently primary trigeminal neuralgia; however some patients may present with clinically classical neuralgia but no vascular conflict on MRI or even at surgery. Several factors have been cited as alternative or supplementary factors that may cause neuralgia. This work focuses on the shape of the petrous ridge at the point of exit from the cavum trigeminus as well as the angulation of the nerve at this point.
Methods
Patients with trigeminal neuralgia that had performed a complete imagery workup according to our protocol and had microvascular decompression were included as well as ten controls. In all subjects, the angle of the petrous ridge as well as the angle of the nerve on passing over the ridge were measured. These were compared from between the neuralgic and the non-neuralgic side and with the measures performed in controls.
Results
In 42 patients, the bony angle of the petrous ridge was measured to be 86° on the neuralgic side, significantly more acute than that of controls (98°, p = 0.004) and with a trend to be more acute than the non-neuralgic side (90°, p = 0.06). The angle of the nerve on the side of the neuralgia was measured to be on average 141°, not significantly different either from the other side (144°, p = 0.2) or from controls (142°, p = 0.4). However, when taking into account the grade of the conflict, the angle was significantly more acute in patients with grade II/III conflict than on the contralateral side, especially when the superior cerebellar artery was the conflicting vessel.
Conclusion
This pilot study analyzes factors other than NVC that may contribute to the pathogenesis of the neuralgia. It appears that aggressive bony edges may contribute—at least indirectly—to the neuralgia. This should be considered for surgical indication and conduct of surgery when patients undergo MVD.




Similar content being viewed by others
References
Cruccu G, Finnerup NB, Jensen TS, Scholz J, Sindou M, Svensson P, Treede R-D, Zakrzewska JM, Nurmikko T (2016) Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology 87:220–228
Dumot C, Sindou M (2015) Trigeminal neuralgia due to neurovascular conflicts from venous origin: an anatomical-surgical study (consecutive series of 124 operated cases). Acta Neurochir 157:455–466
Dumot C, Brinzeu A, Berthiller J, Sindou M (2017) Trigeminal neuralgia due to venous neurovascular conflicts: outcome after microvascular decompression in a series of 55 consecutive patients. Acta Neurochir 159:237–249
Dworkin RH, O’Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, Levy RM, Backonja M, Baron R, Harke H et al (2013) Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 154:2249–2261
Gardner WJ, Dohn DF (1966) Trigeminal neuralgia—hemifacial spasm—Paget’s disease: significance of this association. Brain J Neurol 89:555–562
Gnanalingham K, Joshi SM, Lopez B, Ellamushi H, Hamlyn P (2005) Trigeminal neuralgia secondary to Chiari’s malformation—treatment with ventriculoperitoneal shunt. Surg Neurol 63:586–588 discussion 588-589
Ha SM, Kim SH, Yoo EH, Han I-B, Shin D-A, Cho KG, Chung SS, Park YS (2012) Patients with idiopathic trigeminal neuralgia have a sharper-than-normal trigeminal-pontine angle and trigeminal nerve atrophy. Acta Neurochir 154:1627–1633
Horínek D, Brezová V, Nimsky C, Belsan T, Martinkovic L, Masopust V, Vrána J, Kozler P, Plas J, Krýsl D et al (2009) The MRI volumetry of the posterior fossa and its substructures in trigeminal neuralgia: a validated study. Acta Neurochir 151:669–675
Leal PRL, Hermier M, Froment JC, Souza MA, Cristino-Filho G, Sindou M (2010a) Preoperative demonstration of the neurovascular compression characteristics with special emphasis on the degree of compression, using high-resolution magnetic resonance imaging: a prospective study, with comparison to surgical findings, in 100 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Acta Neurochir 152:817–825
Leal PRL, Froment J-C, Sindou M (2010b) MRI sequences for detection of neurovascular conflicts in patients with trigeminal neuralgia and predictive value for characterization of the conflict (particularly degree of vascular compression). Neurochirurgie 56:43–49
Leal PRL, Hermier M, Souza MA, Cristino-Filho G, Froment JC, Sindou M (2011) Visualization of vascular compression of the trigeminal nerve with high-resolution 3T MRI: a prospective study comparing preoperative imaging analysis to surgical findings in 40 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Neurosurgery 69:15–25 discussion 26
Maarbjerg S, Sørensen MT, Gozalov A, Bendtsen L, Olesen J (2015) Field-testing of the ICHD-3 beta diagnostic criteria for classical trigeminal neuralgia. Cephalalgia Int J Headache 35:291–300
Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G (2017) Trigeminal neuralgia—diagnosis and treatment. Cephalalgia Int J Headache 37:648–657
Mueller HR, Levy A (1963) On the pathogenies of trigeminal neuralgia. Study of mechanical factors by means of craniometry. Acta Neurochir 11:385–397
Parise M, Ribeiro CT, Vincent M, Gasparetto E (2010) Small cerebellopontine angle cisterns inpatients with trigeminal neuralgia. J Neurosurg 112:900–901 author reply 901
Parise M, Acioly MA, Ribeiro CT, Vincent M, Gasparetto EL (2013) The role of the cerebellopontine angle cistern area and trigeminal nerve length in the pathogenesis of trigeminal neuralgia: a prospective case-control study. Acta Neurochir 155:863–868
Rasche D, Kress B, Stippich C, Nennig E, Sartor K, Tronnier VM (2006) Volumetric measurement of the pontomesencephalic cistern in patients with trigeminal neuralgia and healthy controls. Neurosurgery 59:614–620 discussion 614-620
Sindou M, Howeidy T, Acevedo G (2002) Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia (with correlations between topography of pain and site of the neurovascular conflict). Prospective study in a series of 579 patients. Acta Neurochir 144:1–12 discussion 12-13
Sindou M, Leston J, Howeidy T, Decullier E, Chapuis F (2006) Micro-vascular decompression for primary Trigeminal Neuralgia (typical or atypical). Long-term effectiveness on pain; prospective study with survival analysis in a consecutive series of 362 patients. Acta Neurochir 148:1235–1245 discussion 1245
Sindou M, Leston J, Decullier E, Chapuis F (2007) Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg 107:1144–1153
Takada Y, Morimoto T, Sugawara T, Ohno K (2001) Trigeminal neuralgia associated with achondroplasia. Case report with literature review. Acta Neurochir 143:1173–1176
Author information
Authors and Affiliations
Contributions
Andrei Brinzeu: substantial contributions to the conception and design, and acquisition of data, and analysis and interpretation of data; statistical analysis; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. Chloé Dumot: substantial contributions to the acquisition of data and analysis of data, proofing the article, and approval of the final version to be published. Marc Sindou: substantial contributions to the conception and design and interpretation of data, drafting the article, and revising it critically for important intellectual content; revising the article; and approval of the final version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee (Comithee d’ethique de l’etablissement, Hopital Neurologique de Lyon) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
This was an imagery study performed with clinical data collected from patient files collected prospectively. For this type of study, formal consent is not required. All patients, however, gave informed consent for the procedure and the study.
Additional information
Comments
A majority of patients with trigeminal neuralgia have trigeminal root vascular contact on MRI. However, an even larger number of patients without trigeminal neuralgia have asymptomatic vascular contacts on imaging.
This has led to speculations about what extra ingredients are necessary for a vascular contact to cause trigeminal neuralgia, from a genetic predisposition to anatomical features such as cisternal crowding and arachnoidal thickening.
In this well-performed pilot study, the authors add another anatomical variable that might exacerbate the pathological effects of vascular contact. Petrosal bone angulation may tether or stretch the trigeminal root and thereby increase the impact of vessels such as the SCA in initiating trigeminal neuralgia.
The authors correctly point out the study limitations including small sample size and the nature of the control group.
Zvi Harry Rappaport
Petah Tiqva, Israel
Rights and permissions
About this article
Cite this article
Brinzeu, A., Dumot, C. & Sindou, M. Role of the petrous ridge and angulation of the trigeminal nerve in the pathogenesis of trigeminal neuralgia, with implications for microvascular decompression. Acta Neurochir 160, 971–976 (2018). https://doi.org/10.1007/s00701-018-3468-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-018-3468-1