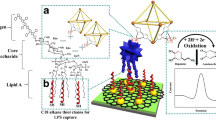
Abstract
A peptide (Li5–025)-modified gold nanoparticle (AuNP)/(titania (TiO2) + 5,10,15,20-tetrakis(4-aminophenyl)-21H,23H-porphine (TAPP))/glassy carbon electrode (GCE) was developed for lipopolysaccharide (LPS) determination. This electrode not only performs well in the electrochemical impedance determination of LPS in serum but can also be easily regenerated under light irradiation. Using Fe(CN)63−/4- as a redox probe, LPS recognition can be indicated by the significantly increased electron-transfer resistance (Ret) as a result of the coaction of the increased steric hindrance from the peptide-LPS complex and the electrostatic repulsion between LPS and Fe(CN)63−/4-. The impedimetric signal was acquired in the frequency range 0.1 Hz ~ 100 kHz with an initial voltage of 174 mV and an amplitude of 10 mV. The resistance changes (ΔRet) are linearly related to the LPS concentrations in a broad range (0.1 pg mL−1 ~ 100 ng mL−1) with a low detection limit (0.08 pg mL−1). Importantly, the electrode shows high selectivity to LPS from Escherichia coli O55:B5 compared to other bacterial sources and considerable anti-interference to 0.1% fetal calf serum, demonstrating its potential application in clinically relevant samples. Another highlight is that the AuNP/(TiO2 + TAPP)/GCE surface can be photocatalytically regenerated under light irradiation (50 mW cm−2, 300–2500 nm) without any obvious damage to the electrode microstructure. After simple peptide re-immobilization, the regenerated electrode demonstrates LPS response similar to the peptide less one, and the deviation is only 2.89% after 5-cycle reuse.

A peptide (Li5–025)-modified AuNP/(TiO2 + TAPP porphine)/GCE was proposed, which not only has excellent electrochemical analytical performances for LPS assay in serum but also can be reused after light irradiation and subsequent peptide re-immobilization.




Similar content being viewed by others
References
Aderem A, Ulevitch RJ (2000) Toll-like receptors in the induction of the innate immune response. Nature 406(6797):782–787
Liu C, Xu J-y, Qi J-f, Liu Y-j, Kang J-w, Wang H-y, Chen S-y, Huang Z, Lu B (2019) A novel nanobiocomposite sandwich immunosensor based on molecularly imprinted nano-membrane for endotoxin detection. Sensors Actuators B Chem 290:1–8
Voss S, Fischer R, Jung G, Wiesmüller K-H, Brock R (2007) A fluorescence-based synthetic LPS sensor. J Am Chem Soc 129(3):554–561
Rangin M, Basu A (2004) Lipopolysaccharide identification with functionalized polydiacetylene liposome sensors. J Am Chem Soc 126(16):5038–5039
Lan M, Wu J, Liu W, Zhang W, Ge J, Zhang H, Sun J, Zhao W, Wang P (2012) Copolythiophene-derived colorimetric and fluorometric sensor for visually supersensitive determination of lipopolysaccharide. J Am Chem Soc 134(15):6685–6694
Kalita P, Dasgupta A, Sritharan V, Gupta S (2015) Nanoparticle–drug bioconjugate as dual functional affinity ligand for rapid point-of-care detection of endotoxin in water and serum. Anal Chem 87(21):11007–11012
Kim S-E, Su W, Cho M, Lee Y, Choe W-S (2012) Harnessing aptamers for electrochemical detection of endotoxin. Anal Biochem 424(1):12–20
Duan Y, Wang N, Huang Z, Dai H, Xu L, Sun S, Ma H, Lin M (2020) Electrochemical endotoxin aptasensor based on a metal-organic framework labeled analytical platform. Mater Sci Eng C 108:110501
Pourmadadi M, Shayeh JS, Omidi M, Yazdian F, Alebouyeh M, Tayebi L (2019) A glassy carbon electrode modified with reduced graphene oxide and gold nanoparticles for electrochemical aptasensing of lipopolysaccharides from Escherichia coli bacteria. Microchim Acta 186(12):787
Ma L, Sun N, Meng Y, Tu C, Cao X, Wei Y, Chu L, Diao A (2018) Harnessing the affinity of magnetic nanoparticles toward dye-labeled DNA and developing it as an universal aptasensor revealed by lipopolysaccharide detection. Anal Chim Acta 1036:107–114
Xu W, Tian J, Shao X, Zhu L, Huang K, Luo Y (2017) A rapid and visual aptasensor for lipopolysaccharides detection based on the bulb-like triplex turn-on switch coupled with HCR-HRP nanostructures. Biosens Bioelectron 89:795–801
Osborne SE, Ellington AD (1997) Nucleic acid selection and the challenge of combinatorial chemistry. Chem Rev 97(2):349–370
Liu F, Mu J, Wu X, Bhattacharjya S, Yeow EKL, Xing B (2014) Peptide–perylene diimide functionalized magnetic nano-platforms for fluorescence turn-on detection and clearance of bacterial lipopolysaccharides. Chem Commun 50(47):6200–6203
Matsumoto M, Horiuchi Y, Yamamoto A, Ochiai M, Niwa M, Takagi T, Omi H, Kobayashi T, Suzuki M-M (2010) Lipopolysaccaride-binding peptides obtained by phage display method. J Microbiol Methods 82(1):54–58
Suzuki MM, Matsumoto M, Yamamoto A, Ochiai M, Horiuchi Y, Niwa M, Omi H, Kobayashi T, Takagi T (2010) Molecular design of LPS-binding peptides. J Microbiol Methods 83(2):153–155
Liu T, Meng F, Cheng W, Sun H, Luo Y, Tang Y, Miao P (2017) Preparation of a peptide-modified electrode for capture and voltammetric determination of endotoxin. ACS Omega 2(6):2469–2473
Hu L, Huo K, Chen R, Gao B, Fu J, Chu PK (2011) Recyclable and high-sensitivity electrochemical biosensing platform composed of carbon-doped TiO2 nanotube arrays. Anal Chem 83(21):8138–8144
Xu J-Q, Liu Y-L, Wang Q, Duo H-H, Zhang X-W, Li Y-T, Huang W-H (2015) Photocatalytically renewable micro-electrochemical sensor for real-time monitoring of cells. Angew Chem Int Ed 54(48):14402–14406
Wang Y-W, Liu Y-L, Xu J-Q, Qin Y, Huang W-H (2018) Stretchable and photocatalytically renewable electrochemical sensor based on sandwich nanonetworks for real-time monitoring of cells. Anal Chem 90(10):5977–5981
Wang X, Caruso RA (2011) Enhancing photocatalytic activity of titania materials by using porous structures and the addition of gold nanoparticles. J Mater Chem 21(1):20–28
Yang L, Sun H, Wang X, Yao W, Zhang W, Jiang L (2019) An aptamer based aggregation assay for the neonicotinoid insecticide acetamiprid using fluorescent upconversion nanoparticles and DNA functionalized gold nanoparticles. Microchim Acta 186(5):308
Yang LM, Han J, Liu W, Li JQ, Jiang L (2015) Conversion of inhibition biosensing to substrate-like biosensing for quinalphos selective detection. Anal Chem 87(10):5270–5277
Wang P, Wang J, Wang X, Yu H, Yu J, Lei M, Wang Y (2013) One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl Catal B 132-133:452–459
Zhang L, Qi H, Zhao Y, Zhong L, Zhang Y, Wang Y, Xue J, Li Y (2019) Au nanoparticle modified three-dimensional network PVA/RGO/TiO2 composite for enhancing visible light photocatalytic performance. Appl Surf Sci 498:143855
Luo X, Morrin A, Killard AJ, Smyth MR (2006) Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 18(4):319–326
Jia J, Wang B, Wu A, Cheng G, Li Z, Dong S (2002) A method to construct a third-generation horseradish peroxidase biosensor: self-assembling gold nanoparticles to three-dimensional sol−gel network. Anal Chem 74(9):2217–2223
Shen W-J, Zhuo Y, Chai Y-Q, Yuan R (2015) Cu-based metal–organic frameworks as a catalyst to construct a ratiometric electrochemical aptasensor for sensitive lipopolysaccharide detection. Anal Chem 87(22):11345–11352
Iturri J, García-Fernández L, Reuning U, García AJ, Ad C, Salierno MJ (2015) Synchronized cell attachment triggered by photo-activatable adhesive ligands allows QCM-based detection of early integrin binding. Sci Rep 5(1):9533
Wu J, Park JP, Dooley K, Cropek DM, West AC, Banta S (2011) Rapid development of new protein biosensors utilizing peptides obtained via phage display. PLoS One 6(10)
Hook F, Kasemo B, Nylander T, Fant C, Sott K, Elwing H (2001) Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: a quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal Chem 73(24):5796–5804
Bhunia A, Mohanram H, Domadia PN, Torres J, Bhattacharjya S (2009) Designed β-boomerang antiendotoxic and antimicrobial peptides: structures and activities in lipopolysaccharide. J Biol Chem 284(33):21991–22004
Ryder MP, Wu X, McKelvey GR, McGuire J, Schilke KF (2014) Binding interactions of bacterial lipopolysaccharide and the cationic amphiphilic peptides polymyxin B and WLBU2. Colloids Surf B 120:81–87
Nileback E, Westberg F, Deinum J, Svedhem S (2010) Viscoelastic sensing of conformational changes in plasminogen induced upon binding of low molecular weight compounds. Anal Chem 82(20):8374–8376
McCubbin GA, Praporski S, Piantavigna S, Knappe D, Hoffmann R, Bowie JH, Separovic F, Martin LL (2011) QCM-D fingerprinting of membrane-active peptides. Eur Biophys J 40(4):437–446
Lu NY, Yang K, Li JL, Yuan B, Ma YQ (2013) Vesicle deposition and subsequent membrane-melittin interactions on different substrates: a QCM-D experiment. Biochim Biophys Acta Biomembr 1828(8):1918–1925
Wang N, Dai H, Sai L, Ma H, Lin M (2019) Copper ion-assisted gold nanoparticle aggregates for electrochemical signal amplification of lipopolysaccharide sensing. Biosens Bioelectron 126:529–534
Shen W-J, Zhuo Y, Chai Y-Q, Yuan R (2016) Ce-based metal-organic frameworks and DNAzyme-assisted recycling as dual signal amplifiers for sensitive electrochemical detection of lipopolysaccharide. Biosens Bioelectron 83:287–292
Xie S, Zhang J, Teng L, Yuan W, Tang Y, Peng Q, Tang Q (2019) Electrochemical detection of lipopolysaccharide based on rolling circle amplification assisted formation of copper nanoparticles for enhanced resistance generation. Sensors Actuators B 301:127072
Lerouge I, Vanderleyden J (2002) O-antigen structural variation: mechanisms and possible roles in animal/plant-microbe interactions. FEMS Microbiol Rev 26(1):17–47
Mazgaeen L, Gurung P (2020) Recent advances in lipopolysaccharide recognition systems. Int J Mol Sci 21(2)
Xia Y, Ye J, Tan K, Wang J, Yang G (2013) Colorimetric visualization of glucose at the submicromole level in serum by a homogenous silver nanoprism–glucose oxidase system. Anal Chem 85(13):6241–6247
McIntyre CA, Reinin G (2009) Reduction in endotoxin levels after performing the prepare for aseptic sort procedure on the BD FACSAria II flow cytometer. BD Biosci Appl Note
Seigel RR, Harder P, Dahint R, Grunze M, Josse F, Mrksich M, Whitesides GM (1997) On-line detection of nonspecific protein adsorption at artificial surfaces. Anal Chem 69(16):3321–3328
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31871878), Shandong Provincial Natural Science Foundation, China (No. ZR2018BC057), the Fundamental Research Funds for the Central Universities (No. 19CX02041A), and Key R&D Program of Shandong Province (No. 2018GSF118032).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 56551 kb)
Rights and permissions
About this article
Cite this article
Yang, L., Gao, Y., Fang, K. et al. Photocatalytically renewable peptide-based electrochemical impedance method for sensing lipopolysaccharide. Microchim Acta 187, 349 (2020). https://doi.org/10.1007/s00604-020-04321-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04321-8