Abstract
Abrin is one of the most toxic phytotoxins to date, and is a potential biological warfare agent. A bio–barcode triggered isothermal amplification for fluorometric determination of abrin is described. Free abrin competes with abrin–coated magnetic microparticles (MMP) probes to bind to gold nanoparticle (AuNP) probes modified with abrin antibody and bio–barcoded DNA. Abundant barcodes are released from the MMP–AuNP complex via dithiothreitol treatment. This triggers an exponential amplification reaction (EXPAR) that is monitored by real–time fluorometry, at typical excitation/emission wavelengths of 495/520 nm. The EXPAR assay is easily operated, highly sensitive and specific. It was used to quantify abrin in spiked commercial samples. The detection limit (at S/N = 3; for n = 6) is 5.6 pg·mL−1 which is considerably lower than previous reports. This assay provides a universal sensing platform and has great potential for determination of various analytes, including small molecules, proteins, DNA, and cells.

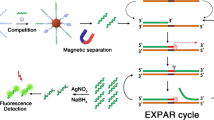
Schematic representation of the bio–barcode triggered exponential amplification reaction (EXPAR) for a fluorometric competitive immunoassay for abrin. The limit of detection is 5.6 pg mL−1 with a large dynamic range from 10 pg mL−1 to 1 µg mL−1.





Similar content being viewed by others
References
Tang JJ, Yu T, Guo L, Xie JW, Shao NS, He ZK (2007) In vitro selection of DNA aptamer against abrin toxin and aptamer–based abrin direct detection. Biosens Bioelectron 22:2456–2463
Cheng J, Lu TH, Liu CL, Lin JY (2010) A biophysical elucidation for less toxicity of agglutinin than abrin–a from the seeds of abrus precatorius in consequence of crystal structure. J Biomed Sci 17:34
Garber EAE, Venkateswaran KV, O'Brien TW (2010) Simultaneous multiplex detection and confirmation of the proteinaceous toxins abrin, ricin, botulinum toxins, and staphylococcus enterotoxins a, B, and C in food. J Agr Food Chem 58:6600–6607
Yang H, Deng M, Ga S, Chen SH, Kang L, Wang JH, Xin WW, Zhang T, You ZR, An Y, Wang JL, Cui DX (2014) Capillary–driven surface–enhanced raman scattering (SERS)–based microfluidic chip for abrin detection. Nanoscale Res Lett 9:138
Dodge AG, Carrasquillo K, Rivera L, Xu L, Wackett LP, Sadowsky MJ (2015) Rapid method using two microbial enzymes for detection of L–abrine in food as a marker for the toxic protein abrin. Appl Environ Microb 81:1610–1615
Cho H, Jaworski J (2014) A portable and chromogenic enzyme–based sensor for detection of abrin poisoning. Biosens Bioelectron 54:667–673
Olsnes S (2004) The history of ricin, abrin and related toxins. Toxicon 44:361–370
Dickers KJ, Bradberry SM, Rice P, Griffiths GD, Vale JA (2003) Abrin poisoning. Toxicol Rev 22:137–142
Li XB, Yang W, Zhang Y, Zhang ZG, Kong T, Li DN, Tang JJ, Liu L, Liu GW, Wang Z (2011) Preparation and identification of monoclonal antibody against abrin–a. J Agr Food Chem 59:9796–9799
Yang W, Li XB, Liu GW, Zhang BB, Zhang Y, Kong T, Tang JJ, Li DN, Wang Z (2011) A colloidal gold probe–based silver enhancement immunochromatographic assay for the rapid detection of abrin–a. Biosens Bioelectron 26:3710–3713
Jie GF, Zhang J, Jie GX, Wang L (2014) A novel quantum dot nanocluster as versatile probe for electrochemiluminescence and electrochemical assays of DNA and cancer cells. Biosens Bioelectron 52:69–75
Hill HD, Mirkin CA (2006) The bio–barcode assay for the detection of protein and nucleic acid targets using DTT–induced ligand exchange. Nat Protoc 1:324–336
Zhang D, Carr DJ, Alocilja EC (2009) Fluorescent bio–barcode DNA assay for the detection of salmonella enterica serovar Enteritidis. Biosens Bioelectron 24:1377–1381
Nam JM, Wise AR, Groves JT (2005) Colorimetric bio–barcode amplification assay for cytokines. Anal Chem 77:6985–6988
Li Y, Liu BW, Li X, Wei QL (2010) Highly sensitive electrochemical detection of human telomerase activity based on bio–barcode method. Biosens Bioelectron 25:2543–2547
Van Ness J, Van Ness LK, Galas DJ (2003) Isothermal reactions for the amplification of oligonucleotides. Proc Natl Acad Sci U S A 100:4504–4509
Shimron S, Wang F, Orbach R, Willner I (2012) Amplified detection of DNA through the enzyme–free autonomous assembly of hemin/G–quadruplex DNAzyme nanowires. Anal Chem 84:1042–1048
Zhang YP, Cui YX, Li XY, Du YC, Tang AN, Kong DM (2019) A modified exponential amplification reaction (EXPAR) with an improved signal–to–noise ratio for ultrasensitive detection of polynucleotide kinase. Chem Commun 55:7611–7614
Chen J, Zhou XQ, Ma YJ, Lin XL, Dai Z, Zou XY (2016) Asymmetric exponential amplification reaction on a toehold/biotin featured template: an ultrasensitive and specific strategy for isothermal microRNAs analysis. Nucleic Acids Res 44:e130
Garber EAE, Walker JL, O'Brien TW (2008) Detection of abrin in food using enzyme–linked immunosorbent assay and electrochemiluminescence technologies. J Food Protect 71:1868–1874
Fu YC, Li PH, Wang T, Bu LJ, Xie Q, Xu XH, Lei LH, Zou C, Chen JH, Yao SZ (2010) Novel polymeric bionanocomposites with catalytic Pt nanoparticles label immobilized for high performance amperometric immunoassay. Biosens Bioelectron 25:1699–1704
Zhou HY, Zhou B, Ma HZ, Carney C, Janda KD (2007) Selection and characterization of human monoclonal antibodies against abrin by phage display. Bioorg Med Chem Lett 17:5690–5692
Jin RC, Wu GS, Li Z, Mirkin CA, Schatz GC (2003) What controls the melting properties of DNA–linked gold nanoparticle assemblies? J Am Chem Soc 125:1643–1654
Jia HX, Li ZP, Liu CH, Cheng YQ (2010) Ultrasensitive detection of microRNAs by exponential isothermal amplification. Angew Chem Int Edit 49:5498–5501
Nie J, Zhang DW, Tie C, Zhou YL, Zhang XX (2014) G–quadruplex based two–stage isothermal exponential amplification reaction for label–free DNA colorimetric detection. Biosens Bioelectron 56:237–242
Ma F, Yang Y, Zhang CY (2014) Ultrasensitive detection of transcription factors using transcription–mediated isothermally exponential amplification–induced chemiluminescence. Anal Chem 86:6006–6011
Zeng ZM, Zhong Y, Yang HC, Fei RH, Zhou R, Luque R, Hu YG (2016) Naturally nano: synthesis of versatile bio–inpired monodisperse microspheres from Bacillus spores and their applications. Green Chem 18:186–196
Zhang M, Huo BY, Yuan S, Ning BA, Bai JL, Peng Y, Liu BL, Gao ZX (2018) Ultrasensitive detection of T–2 toxin in food based on bio–barcode and rolling circle amplification. Anal Chim Acta 1043:98–106
Zhang XQ, Han Q, Ding MY (2015) One–pot synthesis of UiO–66@SiO2 shell–core microspheres as stationary phase for high performance liquid chromatography. RSC Adv 5:1043–1050
Xu Y, Karmakar A, Wang DY, Mahmood MW, Watanabe F, Zhang YB, Fejleh A, Fejleh P, Li ZR, Kannarpady G, Ali S, Biris AR, Biris AS (2010) Multifunctional Fe3O4 cored magnetic–quantum dot fluorescent nanocomposites for RF nanohyperthermia of cancer cells. J Phys Chem 114:5020–5026
Hu JT, Ni PJ, Dai HC, Sun YJ, Wang YL, Jiang S, Li Z (2015) Aptamer–based colorimetric biosensing of abrin using catalytic gold nanoparticles. Analyst 140:3581–3586
Hu KC, Lan DX, Li XM, Zhang SS (2008) Electrochemical DNA biosensor based on nanoporous gold electrode and multifunctional encoded DNA–au bio bar codes. Anal Chem 80:9124–9130
Wang LJ, Zhang Y, Zhang CY (2013) Ultrasensitive detection of telomerase activity at the single–cell level. Anal Chem 85:11509–11517
Zhao J, Yan YL, Zhu L, Li XX, Li GX (2013) An amperometric biosensor for the detection of hydrogen peroxide released from human breast cancer cells. Biosens Bioelectron 41:815–819
Zhang XY, Li Z, Zhou T, Zhou Q, Zeng ZM, Xu XD, Hu YG (2016) A quantum dot–spore nanocomposite pH sensor. Talanta 150:184–189
Homaei AA, Sajedi RH, Sariri R, Seyfzadeh S, Stevanato R (2010) Cysteine enhances activity and stability of immobilized papain. Amino Acids 38:937–942
Shi FP, Wang L, Li Y, Zhang Y, Su XG (2018) A simple “turn–on” detection platform for trypsin activity and inhibitor screening based on N–acetyl–L–cysteine capped CdTe quantum dots. Sensor Actuat B–Chem 255(3):2733–2741
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No. 2017YFC1200903), the Key Research and Development Program of Tianjin (No. 18YFZCNC01260).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare no financial and personal relationships with other people or organizations that can inappropriately influence our work. No professional or other personal interest of any nature or kind in any product, service and/or company can be construed as influencing the position presented in or the review of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1179 kb)
Rights and permissions
About this article
Cite this article
Sun, X., Fei, R., Zhang, L. et al. Bio–barcode triggered isothermal amplification in a fluorometric competitive immunoassay for the phytotoxin abrin. Microchim Acta 187, 127 (2020). https://doi.org/10.1007/s00604-019-3961-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3961-0