Abstract
The authors describe a peroxidase-mimicking nanozyme composed of IrO2 and graphene oxide (GO). It was synthesized from monodisperse IrO2 nanoparticles with an average diameter of 1.7 ± 0.3 nm that were prepared by pulsed laser ablation in ethanol. The nanoparticles were then placed on polyallylamine-modified GO nanosheets through electrostatic interaction. The peroxidase-like activity of the resulting nanocomposites was evaluated by catalytic oxidation of 3,3′,5,5′-tetramethylbenzidine in the presence of H2O2. Kinetic results demonstrated that the catalytic behavior of the nanocomposites follows Michaelis-Menten kinetics. Experiments performed with terephthalic acid and cytochrome C confirmed that the peroxidase-like activity originated from the electron transfer mechanism rather than from generation of hydroxy radicals. The peroxidase-like activity is inhibited in the presence of ascorbic acid (AA). Based on this property, a colorimetric assay was developed for the determination of AA by exploiting the peroxidase-like activity of IrO2/GO nanocomposites. The linear relationship between absorbance at 652 nm and the concentration of AA was acquired. The limit of detection for AA is 324 nM. Further applications of the method for AA detection in real samples were also successfully demonstrated.

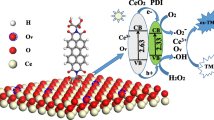
Schematic of the preparation of polyallylamine (PAH)-stabilized IrO2/GO nanocomposites and the colorimetric detection of AA based on the peroxidase-like activity of IrO2/GO nanocomposites.







Similar content being viewed by others
References
Eipper BA, Stoffers DA, Mains RE (1992) The biosynthesis of neuropeptides: peptide alpha amidation. Annu Rev Neurosci 15:57–85. https://doi.org/10.1146/annurev.ne.15.030192.000421
De Rosa S, Cirillo P, Paglia A, Sasso L, Di Palma V, Chiariello M (2010) Reactive oxygen species and antioxidants in the pathophysiology of cardiovascular disease: does the actual knowledge justify a clinical approach? Curr Vasc Pharmacol 8(2):259–275. https://doi.org/10.2174/157016110790887009
Moretti M, Budni J, Freitas AE, Rosa PB, Rodrigues AL (2014) Antidepressant-like effect of ascorbic acid is associated with the modulation of mammalian target of rapamycin pathway. J Psychiatr Res 48(1):16–24. https://doi.org/10.1016/j.jpsychires.2013.10.014
Malashikhina N, Pavlov V (2012) DNA-decorated nanoparticles as nanosensors for rapid detection of ascorbic acid. Biosens Bioelectron 33(1):241–246. https://doi.org/10.1016/j.bios.2012.01.011
Klimczak J, Gliszczyńska-Świgło A (2015) Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem 175:100–105. https://doi.org/10.1016/j.foodchem.2014.11.104
Ma Y, Zhou M, Jin X, Zhang B, Chen H, Guo N (2002) Flow-injection chemiluminescence determination of ascorbic acid by use of the cerium (IV) - Rhodamine B system. Anal Chim Acta 464(2):289–293. https://doi.org/10.1016/S0003-2670(02)00483-X
Gao L, Zhuang J, Nie L, Zhang J, Gu N, Wang T, Feng J, Yang D, Perrett S (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583. https://doi.org/10.1038/nnano.2007.260
Primohamed T, Dowding JM, Wasserman B, Heckert E, Karakoti AS, King JE, Seal S, Self WT (2010) Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun 46(16):2736–2738. https://doi.org/10.1039/B922024K
Liu X, Wang X, Qi C, Han Q, Xiao W, Cai S, Wang C, Yang R (2019) Sensitive colorimetric detection of ascorbic acid using Pt/CeO2 nanocomposites as peroxidase mimics. Appl Surf Sci 479:532–539. https://doi.org/10.1016/j.apsusc.2019.02.135
Cui W, Wang Y, Yang D, Du J (2017) Fluorometric determination of ascorbic acid by exploiting its deactivating effect on the oxidase–mimetic properties of cobalt oxyhydroxide nanosheets. Microchim Acta 184(12):4749–4755. https://doi.org/10.1007/s00604-017-2525-4
Pedone D, Moglianetti M, De Luca E, Bardi G, Pompa PP (2017) Platinum nanoparticles in nanobiomedicine. Chem Soc Rev 46(16):4951–4975. https://doi.org/10.1039/c7cs00152e
Wu G, He S, Peng H, Deng H, Liu A, Lin X, Xia X, Chen W (2014) Citrate-capped platinum nanoparticle as a smart probe for ultrasensitive mercury sensing. Anal Chem 86(21):10955–10960. https://doi.org/10.1021/ac503544w
Liu X, Han Q, Zhang Y, Wang X, Cai S, Wang C, Yang R (2019) Green and facile synthesis of Rh/GO nanocomposites for high catalytic performance. Appl Surf Sci 471:929–934. https://doi.org/10.1016/j.apsusc.2018.12.065
Wang S, Deng W, Yang L, Tan Y, Xie Q, Yao S (2017) Copper-based metal-organic framework nanoparticles with peroxidase-like activity for sensitive colorimetric detection of Staphylococcus aureus. ACS Appl Mater Interfaces 9(29):24440–24445. https://doi.org/10.1021/acsami.7b07307
Wu T, Hou W, Ma Z, Liu M, Liu X, Zhang Y, Yao S (2019) Colorimetric determination of ascorbic acid and the activity of alkaline phosphatase based on the inhibition of the peroxidase-like activity of citric acid-capped Prussian blue nanocubes. Microchim Acta 186(2):123–129. https://doi.org/10.1007/s00604-018-3224-5
Song Y, Qu K, Zhao C, Ren J, Qu X (2010) Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater 22(19):2206–2210. https://doi.org/10.1002/adma.200903783
Chen J, Ge J, Zhang L, Li Z, Li J, Sun Y, Qu L (2016) Reduced graphene oxide nanosheets functionalized with poly(styrene sulfonate) as a peroxidase mimetic in a colorimetric assay for ascorbic acid. Microchim Acta 183(6):1847–1853. https://doi.org/10.1007/s00604-016-1826-3
Othman A, Karimi A, Andreescu S (2016) Functional nanostructures for enzyme based biosensors: properties, fabrication and applications. J Mater Chem B 4(45):7178–7203. https://doi.org/10.1039/c6tb02009g
Amendola V, Meneghetti M (2009) Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys Chem Chem Phys 11(20):3805–3821. https://doi.org/10.1039/b900654k
Jenck JF, Agterberg F, Droescher MJ (2004) Products and processes for a sustainable chemical industry: a review of achievements and prospects. Green Chem 6(11):544–556. https://doi.org/10.1039/b406854h
Zhang D, Gökce B, Barcikowski S (2017) Laser synthesis and processing of colloids: fundamentals and applications. Chem Rev 117(5):3990–4103. https://doi.org/10.1021/acs.chemrev.6b00468
Zhang J, Chaker M, Ma D (2016) Pulsed laser ablation based synthesis of colloidal metal nanoparticles for catalytic applications. J Colloid Interface Sci 489:138–149. https://doi.org/10.1016/j.jcis.2016.07.050
Wagener P, Schwenke A, Barcikowski S (2012) How citrate ligands affect nanoparticle adsorption to microparticle supports. Langmuir 28(14):6132–6140. https://doi.org/10.1021/la204839m
Zhang J, Chen G, Chaker M, Rosei F, Ma D (2013) Gold nanoparticle decorated ceria nanotubes with significantly high catalytic activity for the reduction of nitrophenol and mechanism study. Appl Catal B-Environ 132:107–115. https://doi.org/10.1016/j.apcatb.2012.11.030
Reina G, Gonzálezdomínguez JM, Criado A, Vázquez E, Bianco A, Prato M (2017) Promises, facts and challenges for graphene in biomedical applications. Chem Soc Rev 46(15):4400–4416. https://doi.org/10.1039/c7cs00363c
Sun M, Wang Q, Zou J, Zhang X (2013) Research progress of IrO2 as electrode materials. Electroplat & Finishing 32(10):54–59. https://doi.org/10.3969/j.issn.1004-227X.2013.10.014
Zhang D, Gökce B, Barcikowski S (2017) Laser synthesis and processing of colloids: fundamentals and applications. Chem Rev 117(5):3990–4103. https://doi.org/10.1021/acs.chemrev.6b00468
Kwak I, Kwon IS, Kim J, Park K, Ahn JP, Yoo SJ, Kim JG, Park J (2017) IrO2–ZnO hybrid nanoparticles as highly efficient trifunctional electrocatalysts. J Phys Chem C 121(27):14899–14906. https://doi.org/10.1021/acs.jpcc.7b03844
Kötz R, Neff H, Stucki S (1984) Anodic iridium oxide films: XPS-studies of oxidation state changes and O2 evolution. J Electrochem Soc 131(1):72–77. https://doi.org/10.1149/1.2115548
Cui M, Zhou J, Zhao Y, Song Q (2017) Facile synthesis of iridium nanoparticles with superior peroxidase-like activity for colorimetric determination of H2O2 and xanthine. Sensor Actuat B-Chem 243:203–210. https://doi.org/10.1016/j.snb.2016.11.145
Liu Y, Yu F (2011) Substrate-specific modifications on magnetic iron oxide nanoparticles as an artificial peroxidase for improving sensitivity in glucose detection. Nanotechnology 22(14):145704–145711. https://doi.org/10.1088/0957-4484/22/14/145704
Mu J, Wang Y, Zhao M, Zhang L (2012) Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem Commun 48(19):2540–2542. https://doi.org/10.1039/c2cc17013b
Dong J, Song L, Yin J, He W, Wu Y, Gu N, Zhang Y (2014) Co3O4 nanoparticles with multi-enzyme activities and their application in Immunohistochemical assay. ACS Appl Mater Interfaces 6(3):1959–1970. https://doi.org/10.1021/am405009f
Su H, Liu D, Zhao M, Hu W, Xue S, Cao Q, Le X, Ji L, Mao Z (2015) Dual-enzyme characteristics of Polyvinylpyrrolidone-capped iridium nanoparticles and their cellular protective effect against H2O2-induced oxidative damage. ACS Appl Mater Interfaces 7(15):8233–8242. https://doi.org/10.1021/acsami.5b01271
Acknowledgments
This work was supported by National key research and development program from the Ministry of Science and Technology of China (2016YFC0207102) and National Natural Science Foundation of China (21573050).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2406 kb)
Rights and permissions
About this article
Cite this article
Sun, H., Liu, X., Wang, X. et al. Colorimetric determination of ascorbic acid using a polyallylamine-stabilized IrO2/graphene oxide nanozyme as a peroxidase mimic. Microchim Acta 187, 110 (2020). https://doi.org/10.1007/s00604-019-3897-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3897-4