Abstract
This review (with 99 refs.) summarizes the progress that has been made in colorimetric (i.e. spectrophotometric) determination of organophosphate pesticides (OPPs) using gold and silver nanoparticles (NPs). Following an introduction into the field, a first large section covers the types and functions of organophosphate pesticides. Methods for colorimetric (spectrophotometric) measurements including RGB techniques are discussed next. A further section covers the characteristic features of gold and silver-based NPs. Syntheses and modifications of metal NPs are covered in section 5. This is followed by overviews on enzyme inhibition-based assays, aptamer-based assays and chemical (non-enzymatic) assays, and a discussion of specific features of colorimetric assays. Several Tables are presented that give an overview on the wealth of methods and materials. A concluding section addresses current challenges and discusses potential future trends and opportunities.

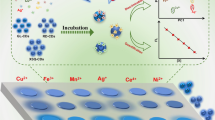
Schematic representation of organophosphate pesticide determinations based on aggregation of nanoparticles (particular silver or gold nanoparticles). This leads to a color change which can be determined visually and monitored by a red shift in the absorption spectrum.














Similar content being viewed by others
References
Bai W, Zhu C, Liu J, Yan M, Yang S, Chen A (2015) Gold nanoparticle–based colorimetric aptasensor for rapid detection of six organophosphorous pesticides. Environ Toxicol Chem 34(10):2244–2249. https://doi.org/10.1002/etc.3088
Chiou J, Leung AHH, Lee HW, W-t W (2015) Rapid testing methods for food contaminants and toxicants. J Integr Agric 14(11):2243–2264. https://doi.org/10.1016/S2095-3119(15)61119-4
Kim K, Tsay OG, Atwood DA, Churchill DG (2011) Destruction and detection of chemical warfare agents. Chem Rev 111(9):5345–5403. https://doi.org/10.1021/cr100193y
Saini RK, Bagri LP, Bajpai AK (2017) Smart nanosensors for pesticide detection. In: Grumezescu AM (ed) New pesticides and soil sensors. Academic Press, pp 519–559. https://doi.org/10.1016/B978-0-12-804299-1.00015-1
Rawtani D, Khatri N, Tyagi S, Pandey G (2018) Nanotechnology-based recent approaches for sensing and remediation of pesticides. J Environ Manag 206:749–762. https://doi.org/10.1016/j.jenvman.2017.11.037
Khatri N, Tyagi S, Rawtani D (2016) Assessment of drinking water quality and its health effects in rural areas of Harij Taluka, Patan District of Northern Gujarat. Environ Claim J 28(3):223–246. https://doi.org/10.1080/10406026.2016.1190249
Kodir A, Imawan C, Permana IS, Handayani W (2016) Pesticide colorimetric sensor based on silver nanoparticles modified by L-cysteine. In: 2016 International Seminar on Sensors, Instrumentation, Measurement and Metrology (ISSIMM), pp 43–47. https://doi.org/10.1109/issimm.2016.7803719
Iyer R, Iken B, Leon A (2015) Developments in alternative treatments for organophosphate poisoning. Toxicol Lett 233(2):200–206. https://doi.org/10.1016/j.toxlet.2015.01.007
Lotti M (2010) Chapter 72 - clinical toxicology of anticholinesterase agents in humans. In: Krieger R (ed) Hayes' handbook of pesticide toxicology, 3rd edn. Academic Press, New York, pp 1543–1589. https://doi.org/10.1016/B978-0-12-374367-1.00072-0
Bala R, Dhingra S, Kumar M, Bansal K, Mittal S, Sharma RK, Wangoo N (2017) Detection of organophosphorus pesticide – Malathion in environmental samples using peptide and aptamer based nanoprobes. Chem Eng J 311:111–116. https://doi.org/10.1016/j.cej.2016.11.070
Eddleston M, Buckley NA, Eyer P, Dawson AH (2008) Management of acute organophosphorus pesticide poisoning. Lancet 371(9612):597–607. https://doi.org/10.1016/S0140-6736(07)61202-1
Verma N, Bhardwaj A (2015) Biosensor Technology for Pesticides—a review. Appl Biochem Biotechnol 175(6):3093–3119. https://doi.org/10.1007/s12010-015-1489-2
Ben Oujji N, Bakas I, Istamboulié G, Ait-Ichou I, Ait-Addi E, Rouillon R, Noguer T (2014) A simple colorimetric enzymatic-assay, based on immobilization of acetylcholinesterase by adsorption, for sensitive detection of organophosphorus insecticides in olive oil. Food Control 46:75–80. https://doi.org/10.1016/j.foodcont.2014.05.006
Stoytcheva M, Zlatev R (2011) Organophosphorus pesticides analysis. In: Pesticides in the modern world-trends in pesticides analysis. IntechOpen, pp 143–164. https://doi.org/10.5772/20892
Kumar DN, Alex SA, Kumar RSS, Chandrasekaran N, Mukherjee A (2015) Acetylcholinesterase inhibition-based ultrasensitive fluorometric detection of malathion using unmodified silver nanoparticles. Colloids Surf A Physicochem Eng Asp 485:111–117. https://doi.org/10.1016/j.colsurfa.2015.09.013
Simonian AL, Good TA, Wang SS, Wild JR (2005) Nanoparticle-based optical biosensors for the direct detection of organophosphate chemical warfare agents and pesticides. Anal Chim Acta 534(1):69–77. https://doi.org/10.1016/j.aca.2004.06.056
Dong L, Craig MM, Khang D, Chen C (2012) Applications of Nanomaterials in biology and medicine. J Nanotechnol 2012:2. https://doi.org/10.1155/2012/816184
Khanna V (2008) Nanoparticle-based sensors. Def Sci J 58(5):608–616. https://doi.org/10.14429/dsj.58.1683
Nanda Kumar D, Satija J, Chandrasekaran N, Mukherjee A (2018) Acetylcholinesterase-based inhibition screening through in situ synthesis of gold nanoparticles: application for detection of nerve agent simulant. J Mol Liq 249:623–628. https://doi.org/10.1016/j.molliq.2017.11.094
Fu G, Chen W, Yue X, Jiang X (2013) Highly sensitive colorimetric detection of organophosphate pesticides using copper catalyzed click chemistry. Talanta 103:110–115. https://doi.org/10.1016/j.talanta.2012.10.016
Rani M, Shanker U (2018) Degradation of traditional and new emerging pesticides in water by nanomaterials: recent trends and future recommendations. Int J Environ Sci Technol 15(6):1347–1380. https://doi.org/10.1007/s13762-017-1512-y
Rapini R, Marrazza G (2016) Biosensor Potential in Pesticide Monitoring. In: Scognamiglio V, Rea G, Arduini F, Palleschi G (eds) Comprehensive analytical chemistry, vol 74. Elsevier, pp 3–31. https://doi.org/10.1016/bs.coac.2016.03.016
Ferri D, Gaviña P, Costero AM, Parra M, Vivancos J-L, Martínez-Máñez R (2014) Detection and discrimination of organophosphorus pesticides in water by using a colorimetric probe array. Sensors Actuators B Chem 202:727–731. https://doi.org/10.1016/j.snb.2014.06.011
Carullo P, Cetrangolo GP, Mandrich L, Manco G, Febbraio F (2015) Fluorescence spectroscopy approaches for the development of a real-time organophosphate detection system using an enzymatic sensor. Sensors 15(2):3932. https://doi.org/10.3390/s150203932
Yüzbaşıoğlu D, Çelik M, Yılmaz S, Ünal F, Aksoy H (2006) Clastogenicity of the fungicide afugan in cultured human lymphocytes. Mutat Res -Gen Tox En 604(1):53–59. https://doi.org/10.1016/j.mrgentox.2006.01.001
Rathnayake LK, Northrup SH (2016) Structure and mode of action of organophosphate pesticides: a computational study. Comput Theor Chem 1088:9–23. https://doi.org/10.1016/j.comptc.2016.04.024
Costero AM, Parra M, Gil S, Gotor R, Martínez-Mañez R, Sancenón F, Royo S (2012) Selective detection of nerve agent simulants by using triarylmethanol-based chromogenic chemodosimeters. Eur J Org Chem 2012(26):4937–4946. https://doi.org/10.1002/ejoc.201200570
Calas A-G, Perche O, Richard O, Perche A, Pâris A, Lauga F, Herzine A, Palomo J, Ardourel M-Y, Menuet A (2016) Characterization of seizures induced by acute exposure to an organophosphate herbicide, glufosinate-ammonium. Neuroreport 27(7):532–541. https://doi.org/10.1097/WNR.0000000000000578
Beuret CJ, Zirulnik F, Giménez MS (2005) Effect of the herbicide glyphosate on liver lipoperoxidation in pregnant rats and their fetuses. Reprod Toxicol 19(4):501–504. https://doi.org/10.1016/j.reprotox.2004.09.009
Guilherme S, Gaivão I, Santos MA, Pacheco M (2012) DNA damage in fish (Anguilla anguilla) exposed to a glyphosate-based herbicide – elucidation of organ-specificity and the role of oxidative stress. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 743(1):1–9. https://doi.org/10.1016/j.mrgentox.2011.10.017
Chaimanee V, Evans JD, Chen Y, Jackson C, Pettis JS (2016) Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. J Insect Physiol 89:1–8. https://doi.org/10.1016/j.jinsphys.2016.03.004
Namba T, Nolte CT, Jackrel J, Grob D (1971) Poisoning due to organophosphate insecticides: acute and chronic manifestations. Am J Med 50(4):475–492. https://doi.org/10.1016/0002-9343(71)90337-8
Murdock RC, Shen L, Griffin DK, Kelley-Loughnane N, Papautsky I, Hagen JA (2013) Optimization of a paper-based ELISA for a human performance biomarker. Anal Chem 85(23):11634–11642. https://doi.org/10.1021/ac403040a
Sabela M, Balme S, Bechelany M, Janot J-M, Bisetty K (2017) A review of gold and silver nanoparticle-based colorimetric sensing assays. Adv Eng Mater 19(12):1700270. https://doi.org/10.1002/adem.201700270
Yan X, Li H, Su X (2018) Review of optical sensors for pesticides. TrAC Trends Anal Chem 103:1–20. https://doi.org/10.1016/j.trac.2018.03.004
Shah P, Zhu X, C-z L (2013) Development of paper-based analytical kit for point-of-care testing. Expert Rev Mol Diagn 13(1):83–91. https://doi.org/10.1586/erm.12.130
Morbioli GG, Mazzu-Nascimento T, Stockton AM, Carrilho E (2017) Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μPADs) - a review. Anal Chim Acta 970:1–22. https://doi.org/10.1016/j.aca.2017.03.037
Kangas MJ, Burks RM, Atwater J, Lukowicz RM, Williams P, Holmes AE (2017) Colorimetric sensor arrays for the detection and identification of chemical weapons and explosives. Crit Rev Anal Chem 47(2):138–153. https://doi.org/10.1080/10408347.2016.1233805
Eksperiandova LP, Khimchenko SV, Stepanenko NA, Shcherbakov IB (2016) Simple instrumental and visual tests for nonlaboratory environmental control. J Anal Methods 2016:9. https://doi.org/10.1155/2016/1270629
Apilux A, Isarankura-Na-Ayudhya C, Tantimongcolwat T, Prachayasittikul V (2015) Paper based acetylcholinesterase inhibition assay combining a wet system for organophosphate and carbamate pesticides detection. EXCLI J 14:307. https://doi.org/10.17179/excli2014-684
Zhao X, Zhao H, Yan L, Li N, Shi J, Jiang C (2019) Recent developments in detection using noble metal nanoparticles. Crit Rev Anal Chem:1–14. https://doi.org/10.1080/10408347.2019.1576496
Alex S, Tiwari A (2015) Functionalized gold nanoparticles: synthesis, properties and applications - a review. J Nanosci Nanotechnol 15(3):1869–1894. https://doi.org/10.1166/jnn.2015.9718
Xiong S, Deng Y, Zhou Y, Gong D, Xu Y, Yang L, Chen H, Chen L, Song T, Luo A, Deng X, Zhang C, Jiang Z (2018) Current progress in biosensors for organophosphorus pesticides based on enzyme functionalized nanostructures: a review. Anal Methods 10(46):5468–5479. https://doi.org/10.1039/c8ay01851k
Jazayeri MH, Aghaie T, Avan A, Vatankhah A, Ghaffari MRS (2018) Colorimetric detection based on gold nano particles (GNPs): an easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion). Sens Biosensing Res 20:1–8. https://doi.org/10.1016/j.sbsr.2018.05.002
Mancuso M, Jiang L, Cesarman E, Erickson D (2013) Multiplexed colorimetric detection of Kaposi's sarcoma associated herpesvirus and Bartonella DNA using gold and silver nanoparticles. Nanoscale 5(4):1678–1686. https://doi.org/10.1039/c3nr33492a
Khan I, Saeed K, Khan I (2017) Nanoparticles: properties, applications and toxicities. Arab J Chem. https://doi.org/10.1016/j.arabjc.2017.05.011
Njoki PN, Lim I-IS, Mott D, Park H-Y, Khan B, Mishra S, Sujakumar R, Luo J, Zhong C-J (2007) Size correlation of optical and spectroscopic properties for gold nanoparticles. J Phys Chem C 111(40):14664–14669. https://doi.org/10.1021/jp074902z
Barman G, Maiti S, Konar Laha J (2013) Trichloroacetic acid assisted synthesis of gold nanoparticles and its application in detection and estimation of pesticide. J Anal Sci Technol 4(1):3. https://doi.org/10.1186/2093-3371-4-3
Halas NJ, Lal S, Chang W-S, Link S, Nordlander P (2011) Plasmons in strongly coupled metallic nanostructures. Chem Rev 111(6):3913–3961. https://doi.org/10.1021/cr200061k
Sepúlveda B, Angelomé PC, Lechuga LM, Liz-Marzán LM (2009) LSPR-based nanobiosensors. Nano Today 4(3):244–251. https://doi.org/10.1016/j.nantod.2009.04.001
Nath S, Jana S, Pradhan M, Pal T (2010) Ligand-stabilized metal nanoparticles in organic solvent. J Colloid Interface Sci 341(2):333–352. https://doi.org/10.1016/j.jcis.2009.09.049
Zewde B, Ambaye A, Stubbs J III, Raghavan D (2016) A review of stabilized silver nanoparticles—synthesis, biological properties, characterization, and potential areas of applications. Nanotechnol Nanomed 4(1043):1–14
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382(6592):607–609. https://doi.org/10.1038/382607a0
Chang D, Zakaria S, Deng M, Allen N, Tram K, Li Y (2016) Integrating Deoxyribozymes into colorimetric sensing platforms. Sensors 16(12):2061. https://doi.org/10.3390/s16122061
Piriya VSA, Joseph P, Daniel SCGK, Lakshmanan S, Kinoshita T, Muthusamy S (2017) Colorimetric sensors for rapid detection of various analytes. Mater Sci Eng C 78:1231–1245. https://doi.org/10.1016/j.msec.2017.05.018
Kumar P, Kim K-H, Deep A (2015) Recent advancements in sensing techniques based on functional materials for organophosphate pesticides. Biosens Bioelectron 70:469–481. https://doi.org/10.1016/j.bios.2015.03.066
Li H, Guo J, Ping H, Liu L, Zhang M, Guan F, Sun C, Zhang Q (2011) Visual detection of organophosphorus pesticides represented by mathamidophos using au nanoparticles as colorimetric probe. Talanta 87:93–99. https://doi.org/10.1016/j.talanta.2011.09.046
Štěpánková Š, Vorčáková K (2016) Cholinesterase-based biosensors. J Enzym Inhib Med Ch 31(sup3):180–193. https://doi.org/10.1080/14756366.2016.1204609
Satnami ML, Korram J, Nagwanshi R, Vaishanav SK, Karbhal I, Dewangan HK, Ghosh KK (2018) Gold nanoprobe for inhibition and reactivation of acetylcholinesterase: an application to detection of organophosphorus pesticides. Sensors Actuators B Chem 267:155–164. https://doi.org/10.1016/j.snb.2018.03.181
Bala R, Sharma RK, Wangoo N (2015) Highly sensitive colorimetric detection of ethyl parathion using gold nanoprobes. Sensors Actuators B Chem 210:425–430. https://doi.org/10.1016/j.snb.2014.12.123
Pavlov V, Xiao Y, Willner I (2005) Inhibition of the acetycholine esterase-stimulated growth of au nanoparticles: nanotechnology-based sensing of nerve gases. Nano Lett 5(4):649–653. https://doi.org/10.1021/nl050054c
Sun J, Guo L, Bao Y, Xie J (2011) A simple, label-free AuNPs-based colorimetric ultrasensitive detection of nerve agents and highly toxic organophosphate pesticide. Biosens Bioelectron 28(1):152–157. https://doi.org/10.1016/j.bios.2011.07.012
Liu D, Chen W, Wei J, Li X, Wang Z, Jiang X (2012) A highly sensitive, dual-readout assay based on gold nanoparticles for organophosphorus and carbamate pesticides. Anal Chem 84(9):4185–4191. https://doi.org/10.1021/ac300545p
Wu S, Li D, Wang J, Zhao Y, Dong S, Wang X (2017) Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sensors Actuators B Chem 238:427–433. https://doi.org/10.1016/j.snb.2016.07.067
Lu L, Xia Y (2015) Enzymatic reaction modulated gold nanorod end-to-end self-assembly for ultrahigh sensitively colorimetric sensing of cholinesterase and organophosphate pesticides in human blood. Anal Chem 87(16):8584–8591. https://doi.org/10.1021/acs.analchem.5b02516
Virel A, Saa L, Pavlov V (2009) Modulated growth of nanoparticles. Application for sensing nerve gases. Anal Chem 81(1):268–272. https://doi.org/10.1021/ac801949x
Tan MJ, Hong Z-Y, Chang M-H, Liu C-C, Cheng H-F, Loh XJ, Chen C-H, Liao C-D, Kong KV (2017) Metal carbonyl-gold nanoparticle conjugates for highly sensitive SERS detection of organophosphorus pesticides. Biosens Bioelectron 96:167–172. https://doi.org/10.1016/j.bios.2017.05.005
Li Z, Wang Y, Ni Y, Kokot S (2014) Unmodified silver nanoparticles for rapid analysis of the organophosphorus pesticide, dipterex, often found in different waters. Sensors Actuators B Chem 193:205–211. https://doi.org/10.1016/j.snb.2013.11.096
Lv B, Wei M, Liu Y, Liu X, Wei W, Liu S (2016) Ultrasensitive photometric and visual determination of organophosphorus pesticides based on the inhibition of enzyme-triggered formation of core-shell gold-silver nanoparticles. Microchim Acta 183(11):2941–2948. https://doi.org/10.1007/s00604-016-1939-8
Kim YS, Kim JH, Kim IA, Lee SJ, Jurng J, Gu MB (2010) A novel colorimetric aptasensor using gold nanoparticle for a highly sensitive and specific detection of oxytetracycline. Biosens Bioelectron 26(4):1644–1649. https://doi.org/10.1016/j.bios.2010.08.046
Bala R, Kumar M, Bansal K, Sharma RK, Wangoo N (2016) Ultrasensitive aptamer biosensor for malathion detection based on cationic polymer and gold nanoparticles. Biosens Bioelectron 85:445–449. https://doi.org/10.1016/j.bios.2016.05.042
Wang P, Wan Y, Ali A, Deng S, Su Y, Fan C, Yang S (2016) Aptamer-wrapped gold nanoparticles for the colorimetric detection of omethoate. SCIENCE CHINA Chem 59(2):237–242. https://doi.org/10.1007/s11426-015-5488-5
Abnous K, Danesh NM, Ramezani M, Alibolandi M, Emrani AS, Lavaee P, Taghdisi SM (2018) A colorimetric gold nanoparticle aggregation assay for malathion based on target-induced hairpin structure assembly of complementary strands of aptamer. Microchim Acta 185(4):216. https://doi.org/10.1007/s00604-018-2752-3
Lisha KP, Anshup PT (2009) Enhanced visual detection of pesticides using gold nanoparticles. J Environ Sci Health B 44(7):697–705. https://doi.org/10.1080/03601230903163814
Kiran K (2013) Detection of chlorpyrifos pesticide in various water samples using gold nanoparticles. J Res Eng Technol 2(11):2319–2322
Newman JDS, Roberts JM, Blanchard GJ (2007) Optical organophosphate/phosphonate sensor based upon gold nanoparticle functionalized quartz. Anal Chim Acta 602(1):101–107. https://doi.org/10.1016/j.aca.2007.08.051
Fahimi-Kashani N, Hormozi-Nezhad MR (2016) Gold-nanoparticle-based colorimetric sensor array for discrimination of organophosphate pesticides. Anal Chem 88(16):8099–8106. https://doi.org/10.1021/acs.analchem.6b01616
D'Souza SL, Pati RK, Kailasa SK (2014) Ascorbic acid functionalized gold nanoparticles as a probe for colorimetric and visual read-out determination of dichlorvos in environmental samples. Anal Methods 6(22):9007–9014. https://doi.org/10.1039/c4ay01004c
Park Y, Im AR, Hong YN, Kim C-K, Kim YS (2011) Detection of malathion, fenthion and methidathion by using heparin-reduced gold nanoparticles. J Nanosci Nanotechnol 11(9):7570–7578. https://doi.org/10.1166/jnn.2011.5123
Rohit JV, Basu H, Singhal RK, Kailasa SK (2016) Development of p-nitroaniline dithiocarbamate capped gold nanoparticles-based microvolume UV–vis spectrometric method for facile and selective detection of quinalphos insecticide in environmental samples. Sensors Actuators B Chem 237:826–835. https://doi.org/10.1016/j.snb.2016.07.019
Ma S, He J, Guo M, Sun X, Zheng M, Wang Y (2018) Ultrasensitive colorimetric detection of triazophos based on the aggregation of silver nanoparticles. Colloids Surf A Physicochem Eng Asp 538:343–349. https://doi.org/10.1016/j.colsurfa.2017.11.030
Wang X, Yang Y, Dong J, Bei F, Ai S (2014) Lanthanum-functionalized gold nanoparticles for coordination–bonding recognition and colorimetric detection of methyl parathion with high sensitivity. Sensors Actuators B Chem 204:119–124. https://doi.org/10.1016/j.snb.2014.07.093
Li X, Cui H, Zeng Z (2018) A simple colorimetric and fluorescent sensor to detect organophosphate pesticides based on adenosine triphosphate-modified gold nanoparticles. Sensors 18(12):4302. https://doi.org/10.3390/s18124302
Kim MS, Kim GW, Park TJ (2015) A facile and sensitive detection of organophosphorus chemicals by rapid aggregation of gold nanoparticles using organic compounds. Biosens Bioelectron 67:408–412. https://doi.org/10.1016/j.bios.2014.08.073
Bhamore JR, Ganguly P, Kailasa SK (2016) Molecular assembly of 3-mercaptopropinonic acid and guanidine acetic acid on silver nanoparticles for selective colorimetric detection of triazophos in water and food samples. Sensors Actuators B Chem 233:486–495. https://doi.org/10.1016/j.snb.2016.04.111
Xiong D, Li H (2008) Colorimetric detection of pesticides based on calixarene modified silver nanoparticles in water. Nanotechnology 19(46):465502. https://doi.org/10.1088/0957-4484/19/46/465502
McMahon G, O’Malley S, Nolan K, Diamond D (2003) Important calixarene derivatives - their synthesis and applications. Arkivoc 7:23–31. https://doi.org/10.3998/ark.5550190.0004.704
Acharya A, Samanta K, Rao CP (2012) Conjugates of calixarenes emerging as molecular entities of nanoscience. Coord Chem Rev 256(17):2096–2125. https://doi.org/10.1016/j.ccr.2012.05.018
Kongor AR, Mehta VA, Modi KM, Panchal MK, Dey SA, Panchal US, Jain VK (2016) Calix-based nanoparticles: a review. Top Curr Chem 374(3):28. https://doi.org/10.1007/s41061-016-0029-z
Liu G, Yang X, Li T, Yu H, Du X, She Y, Wang J, Wang S, Jin F, Jin M, Shao H, Zheng L, Zhang Y, Zhou P (2015) Spectrophotometric and visual detection of the herbicide atrazine by exploiting hydrogen bond-induced aggregation of melamine-modified gold nanoparticles. Microchim Acta 182(11–12):1983–1989. https://doi.org/10.1007/s00604-015-1531-7
Guo L, Xu Y, Ferhan AR, Chen G, Kim D-H (2013) Oriented gold nanoparticle aggregation for colorimetric sensors with surprisingly high analytical figures of merit. J Am Chem Soc 135(33):12338–12345. https://doi.org/10.1021/ja405371g
Lin C-Y, Yu C-J, Lin Y-H, Tseng W-L (2010) Colorimetric sensing of silver(I) and mercury(II) ions based on an assembly of tween 20-stabilized gold nanoparticles. Anal Chem 82(16):6830–6837. https://doi.org/10.1021/ac1007909
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Martinez AW, Phillips ST, Carrilho E, Thomas SW III, Sindi H, Whitesides GM (2008) Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem 80(10):3699–3707. https://doi.org/10.1021/ac800112r
Thanh NTK, Rosenzweig Z (2002) Development of an aggregation-based immunoassay for anti-protein a using gold nanoparticles. Anal Chem 74(7):1624–1628. https://doi.org/10.1021/ac011127p
Jornet-Martínez N, Gómez-Ojea R, Tomás-Huercio O, Herráez-Hernández R, Campíns-Falcó P (2018) Colorimetric determination of alcohols in spirit drinks using a reversible solid sensor. Food Control 94:7–16. https://doi.org/10.1016/j.foodcont.2018.06.020
Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL (1998) One-pot colorimetric differentiation of polynucleotides with Single Base imperfections using gold nanoparticle probes. J Am Chem Soc 120(9):1959–1964. https://doi.org/10.1021/ja972332i
Hutter E, Maysinger D (2013) Gold-nanoparticle-based biosensors for detection of enzyme activity. Trends Pharmacol Sci 34(9):497–507. https://doi.org/10.1016/j.tips.2013.07.002
Free P, Shaw CP, Lévy R (2009) PEGylation modulates the interfacial kinetics of proteases on peptide-capped gold nanoparticles. Chem Commun 33:5009–5011. https://doi.org/10.1039/b910657j
Acknowledgements
The authors would like to highly acknowledge financial support (UPNM/2018/CHEMDEF/ST/2) from Ministry of Education Malaysia. The authors also gratefully acknowledge Universiti Pertahanan Nasional Malaysia (National Defence University of Malaysia).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Che Sulaiman, I.S., Chieng, B.W., Osman, M.J. et al. A review on colorimetric methods for determination of organophosphate pesticides using gold and silver nanoparticles. Microchim Acta 187, 131 (2020). https://doi.org/10.1007/s00604-019-3893-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3893-8