Abstract
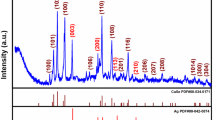
This work describes the synthesis of a nanocomposite consisting of Ag2O, silver nanoparticles and N,S-doped carbon quantum dots (Ag2O/Ag@NS-CQD). The NS-CQD were prepared by hydrothermal treatment of p-aminobenzenesulfonic acid. They act as both the reducing and stabilizing agent for synthesis of Ag2O/Ag@NS-CQD. The composite was characterized by UV-vis spectroscopy, transmission electron microscopy, X-ray diffraction and X-ray photoelectron spectroscopy. The glassy carbon electrode (GCE) was modified by coating it with Ag2O/Ag@NS-CQD. It exhibits excellent amperometric response to catechol, typically at a low working potential of around 0.25 V. Under the best experimental conditions, the sensor has a wide linear response (0.2 to 180 μM) and a low detection limit (13 nM; at S/N = 3). The method was applied to analysis of spiked water samples and gave satisfactory results.

Schematic representation of the preparation of the Ag/Ag2O@N,S-doped carbon quantum dots composite using p-aminobenzenesulfonic acid and silver nitrate as the starting materials. The corresponding modified glassy carbon electrode exhibits the excellent amperometric sensing performance toward catechol at pH 7.0 with low detection limit and good selectivity.







Similar content being viewed by others
References
Palanisamy S, Ramaraj SK, Chen SM, Chiu TW, Velusamy V, Yang TCK, Chen TW, Selvam S (2017) One pot electrochemical synthesis of poly(melamine) entrapped gold nanoparticles composite for sensitive and low level detection of catechol. J Colloid Interface Sci 496:364–370
Chen Y, Liu XY, Zhang S, Yang LQ, Liu ML, Zhang YY, Yao SZ (2017) Ultrasensitive and simultaneous detection of hydroquinone, catechol and resorcinol based on the electrochemical co-reduction prepared au-Pd nanoflower/reduced graphene oxide nanocomposite. Electrochim Acta 231:677–685
Palanisamy S, Thangavelu K, Chen SM, Thirumalraj B, Liu XH (2016) Preparation and characterization of gold nanoparticles decorated on graphene oxide@polydopamine composite: application for sensitive and low potential detection of catechol. Sensors Actuators B Chem 233:298–306
Rao HB, Liu YT, Zhong J, Zhang ZY, Zhao X, Liu X, Jiang YY, Zou P, Wang XX, Wang YY (2017) Gold nanoparticle/chitosan@N,S co-doped multiwalled carbon nanotubes sensor: fabrication, characterization, and electrochemical detection of catechol and nitrite. ACS Sustain Chem Eng 5:10926–10939
Huang YH, Chen JH, Ling LJ, Su ZB, Sun X, Hu SR, Weng W, Huang Y, Wu WB, He YS (2015) Simultaneous electrochemical detection of catechol and hydroquinone based on gold nanoparticles@carbon nanocages modified electrode. Analyst 140:7939–7947
Tashkhourian J, Daneshi M, Nami-Ana F, Behbahani M, Bagheri A (2016) Simultaneous determination of hydroquinone and catechol at gold nanoparticles mesoporous silica modified carbon paste electrode. J Hazard Mater 318:117–124
Wang HL, Hu QQ, Meng Y, Jin ZE, Fang ZL, Fu QR, Gao WH, Xu L, Song YB, Lu FS (2018) Efficient detection of hazardous catechol and hydroquinone with MOF-rGO modified carbon paste electrode. J Hazard Mater 353:151–157
Sharma VV, Gualandi I, Vlamidis Y, Tonelli D (2017) Electrochemical behavior of reduced graphene oxide and multi-walled carbon nanotubes composites for catechol and dopamine oxidation. Electrochim Acta 246:415–423
Chen S, Hai X, Chen XW, Wang JH (2014) In situ growth of silver nanoparticles on graphene quantum dots for ultrasensitive colorimetric detection of H2O2 and glucose. Anal Chem 86:6689–6694
Pathak PK, Kumar A, Prasad BB (2019) Functionalized nitrogen doped graphene quantum dots and bimetallic au/ag core-shell decorated imprinted polymer for electrochemical sensing of anticancerous hydroxyurea. Biosens Bioelectron 127:10–18
Bhanjana G, Chaudhary GR, Dilbaghi N, Chauhan M, Kim KH, Kumar S (2019) Novel electrochemical sensor for mononitrotoluenes using silver oxide quantum dots. Electrochim Acta 293:283–289
Gallardo OAD, Moiraghi R, Macchione MA, Godoy JA, Perez MA, Coronado EA, Macagno VA (2012) Silver oxide particles/silver nanoparticles interconversion: susceptibility of forward/backward reactions to the chemical environment at room temperature. RSC Adv 2:2923–2929
Tufa LT, Oh S, Tran VT, Kim J, Jeong KJ, Park TJ, Kim HJ, Lee J (2018) Electrochemical immunosensor using nanotriplex of graphene quantum dots, Fe3O4, and ag nanoparticles for tuberculosis. Electrochim Acta 290:369–377
Liao S, Zhao XY, Zhu FW, Chen M, Wu ZL, Song XZ, Yang H, Chen XQ (2018) Novel S, N-doped carbon quantum dot-based "off-on" fluorescent sensor for silver ion and cysteine. Talanta 180:300–308
Buk V, Pemble ME, Twomey K (2018) Fabrication and evaluation of a carbon quantum dot/gold nanoparticle nanohybrid material integrated onto planar micro gold electrodes for potential bioelectrochemical sensing applications. Electrochim Acta 293:307–317
Shen LM, Chen Q, Sun ZY, Chen XW, Wang JH (2014) Assay of biothiols by regulating the growth of silver nanoparticles with C-dots as reducing agent. Anal Chem 86:5002–5008
Chen JB, Che HN, Huang K, Liu CB, Shi WD (2016) Fabrication of a ternary plasmonic photocatalyst CQDs/ag/Ag2O to harness charge flow for photocatalytic elimination of pollutants. Appl Catal B 192:134–144
Amjadi M, Hallaj T, Asadollahi H, Song ZL, de Frutos M, Hildebrandt N (2017) Facile synthesis of carbon quantum dot/silver nanocomposite and its application for colorimetric detection of methimazole. Sensors Actuators B Chem 244:425–432
Hu CC, Wang MS, Hung WZ (2017) Influence of solvothermal synthesis on the photocatalytic degradation activity of carbon nitride under visible light irradiation. Chem Eng Sci 167:1–9
Yang YM, Liu NY, Qiao S, Liu RH, Huang H, Liu Y (2015) Silver modified carbon quantum dots for solvent-free selective oxidation of cyclohexane. New J Chem 39:2815–2821
Xia XY, Deng N, Cui GW, Xie JF, Shi XF, Zhao YQ, Wang Q, Wang W, Tang B (2015) NIR light induced H2 evolution by a metal-free photocatalyst. Chem Commun 51:10899–10902
Liu H, Sun P, Feng MB, Liu HX, Yang SG, Wang LS, Wang ZY (2016) Nitrogen and sulfur co-doped CNT-COOH as an efficient metal-free catalyst for the degradation of UV filter BP-4 based on sulfate radicals. Appl Catal B 187:1–10
Zhan TR, Lu SS, Liu XL, Teng HN, Hou WG (2018) Alginate derived Co3O4/co nanoparticles decorated in N-doped porous carbon as an efficient bifunctional catalyst for oxygen evolution and reduction reactions. Electrochim Acta 265:681–689
Sun W, Xi MY, Zhang L, Zhan TR, Gao HW, Jiao KW (2011) Electrochemical behaviors of thymine on a new ionic liquid modified carbon electrode and its detection. Electrochim Acta 56:222–226
Zhan TR, Tan ZW, Tian X, Hou WG (2017) Ionic liquid functionalized graphene oxide-au nanoparticles assembly for fabrication of electrochemical 2,4-Dichlorophenol sensor. Sens. Actuators B 246:638–646
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Beitollahi H, Karimi-Maleh H, Khabazzadeh H (2008) Nanomolar and selective determination of epinephrine in the presence of norepinephrine using carbon paste electrode modified with carbon nanotubes and novel 2-(4-oxo-3-phenyl-3,4-dihydro-quinazolinyl)-N'-phenyl-hydrazinecarbothioamide. Anal Chem 80:9848–9851
Oyama N, Anson FC (1980) Catalysis of electrode processes by multiply-charged metal complexes electrostatically bound to polyelectrolyte coatings on graphite electrodes, and the use of polymer-coated rotating disk electrodes in diagnosing kinetic and conduction mechanisms. Anal Chem 52:1192–1198
Zhang YZ, Sun RX, Luo BM, Wang LJ (2015) Boron-doped graphene as high-performance electrocatalyst for the simultaneously electrochemical determination of hydroquinone and catechol. Electrochim Acta 156:228–234
Du HJ, Ye JS, Zhang JQ, Huang XD, Yu CZ (2011) A voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone. J Electroanal Chem 650:209–213
Ma XM, Liu ZN, Qiu CC, Chen T, Ma HY (2013) Simultaneous determination of hydroquinone and catechol based on glassy carbon electrode modified with gold-graphene nanocomposite. Microchim Acta 180:461–468
Zhang YL, Xie JL, Xiao SX, Yang ZM, Pang PF, Gao YT (2014) Simultaneous electrochemical determination of catechol and hydroquinone based on graphene-TiO2, nanocomposite modified glassy carbon electrode. Sensors Actuators B Chem 204:102–108
Huang YH, Chen JH, Sun X, Su ZB, Xing HT, Hu SR, Weng W, Guo HX, Wu WB, He YS (2009) One-pot hydrothermal synthesis carbon nanocages-reduced graphene oxide composites for simultaneous electrochemical detection of catechol and hydroquinone. Sensors Actuators B Chem 212:165–173
Acknowledgments
We are grateful to the Natural Science Foundation of Shandong Province, China (No. ZR2019MB062, ZR2014JL013, JQ201704), the Key Research and Development Program of Shandong Province (2017GGX20143), Taishan Scholar Program of Shandong Province of China (No. ts201712045), the Foundation of Key Laboratory of Sensor Analysis of Tumor Marker, Ministry of Education, QUST (SATM201603), the foundation of Key Laboratory of Colloid and Interface Chemistry (Shandong University), Ministry of Education (201702), the open foundation from the Key Lab of Marine Bioactive Substance and Modern Analysis Technology, SOA (MBSMAT-2017-02, MBSMAT-2016-02 and MBSMAT-2015-04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1152 kb)
Rights and permissions
About this article
Cite this article
Zhan, T., Ding, G., Cao, W. et al. Amperometric sensing of catechol by using a nanocomposite prepared from Ag/Ag2O nanoparticles and N,S-doped carbon quantum dots. Microchim Acta 186, 743 (2019). https://doi.org/10.1007/s00604-019-3848-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3848-0