Abstract
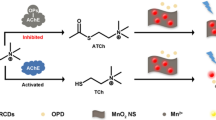
Nitrogen and chlorine dually-doped carbon dots (N,Cl-CDs) were hydrothermally prepared starting from 4-chloro-1,2-diaminobenzene and dopamine. The N,Cl-CDs exhibit strong orange fluorescence, with excitation/emission maxima at 420/570 nm and a relative high quantum yield (15%). The N,Cl-CDs were employed to detect acetylcholinesterase (AChE) activity and organophosphate pesticides (OPs) which are enzyme inhibitors. Acetylthiocholine is enzymatically split by AChE to produce thiocholine which triggers the decomposition of Ellmans’s reagent to form a yellow colored product (2-nitro-5-thiobenzoate anion). The product causes an inner filter effect (IEF) on the fluorescence of the N,Cl-CDs. Fluorescence decreases linearly in the 0.017 to 5.0 Unit·L−1 AChE activity range, and the detection limit is 2 mUnit·L−1. If organophosphates are present, the activity of AChE becomes increasingly blocked, and this leads to a less expressed IFE and an increasing recovery of fluorescence. This was used for the quantification of OPs. Response is linear in the 0.3–1000 μg·L−1 OP concentration range with a 30 ng·L−1 detection limit.

Schematic representation of the synthesis of nitrogen and chlorine dually-doped carbon dots (N,Cl-CDs) and the recognition of organophosphate pesticides by N,Cl-CDs.





Similar content being viewed by others
References
Feng FD, Tang YL, Wang S, Li Y, Zhu D (2007) Continuous fluorometric assays for acetylcholinesterase activity and inhibition with conjugated polyelectrolytes. Angew Chem Int Ed 46:7882–7886
Zhang Y, Cai Y, Qi Z, Lu L, Qian Y (2013) DNA-templated silver nanoclusters for fluorescence turn-on assay of acetylcholinesterase activity. Anal Chem 85:8455–8461
Wang M, Gu XG, Zhang GX, Zhang D, Zhu D (2009) Convenient and continuous fluorometric assay method for acetylcholinesterase and inhibitor screening based on the aggregationinduced emission. Anal Chem 81:4444–4449
Chen J, Liao DL, Wang Y, Zhou H, Li W, Yu C (2013) Real-time fluorometric assay for acetylcholinesterase activity and inhibitor screening through the pyrene probe monomer-excimer transition. Org Lett 15:2132–2135
Lei C, Wang Z, Nie Z, Deng H, Hu H, Huang Y, Yao S (2015) Resurfaced fluorescent protein as a sensing platform for label-free detection of copper(II) ion and acetylcholinesterase activity. Anal Chem 87:1974–1980
Yi YH, Zhu GB, Liu C, Huang Y, Zhang Y, Li H, Zhao J, Yao S (2013) A label-free silicon quantum dots-based photoluminescence sensor for ultrasensitive detection of pesticides. Anal Chem 85:11464–11470
Liu DB, Chen WW, Wei JH, Li X, Wang Z, Jiang X (2012) A highly sensitive, dual-readout assay based on gold nanoparticles for organophosphorus and carbamate pesticides. Anal Chem 84:4185–4191
Gill R, Bahshi L, Freeman R, Willner I (2008) Optical detection of glucose and acetylcholine esterase inhibitors by H2O2-sensitive CdSe/ZnS quantum dots. Angew Chem Int Ed 47:1676–1679
Zhang N, Si Y, Sun Z, Li S, Li S, Lin Y, Wang H (2014) Lab-on-a-drop: biocompatible fluorescent nanoprobes of gold nanoclusters for label-free evaluation of phosphorylation-induced inhibition of acetylcholinesterase activity towards the ultrasensitive detection of pesticide residues. Analyst 139:4620–4628
Sun J, Yang X (2015) Gold nanoclusters-Cu(2+) ensemble-based fluorescence turn-on and real-time assay for acetylcholinesterase activity and inhibitor screening. Biosens Bioelectron 74:177–182
Lu X, Li YS, Tao L et al (2018) Amorphous metal boride as a novel platform for acetylcholinesterase biosensor development and detection of organophosphate pesticides. Nanotechnology 30:5
Su YS, Jen JF (2010) Determination of organophosphorous pesticides in water using in-syringe ultrasound-assisted emulsification and gas chromatography with electron-capture detection. J Chromatogr A 1217:5043–5049
Abdollahzadeh Y, Yamini Y, Jabbari A, Esrafili A, Rezaee M (2012) Application of ultrasound-assisted emulsification microextraction followed by gas chromatography for determination of organophosphorus pesticides in water and soil samples. Anal Methods 4:830
He LJ, Luo XL, Jiang XM, Qu L (2010) A new 1,3-dibutylimidazolium hexafluorophosphate ionic liquid-based dispersive liquid-liquid microextraction to determine organophosphorus pesticides in water and fruit samples by high-performance liquid chromatography. J Chromatogr A 1217:5013–5020
Huang GM, Ouyang J, Baeyens WRG, Yang Y, Tao C (2002) High-performance liquid chromatographic assay of dichlorvos, isocarbophos and methyl parathion from plant leaves using chemiluminescence detection. Anal Chim Acta 474:21–29
Zhang K, Mei QS, Guan GJ, Liu B, Wang S, Zhang Z (2010) Ligand replacement-induced fluorescence switch of quantum dots for ultrasensitive detection of organophosphorothioate pesticides. Anal Chem 82:9579–9586
Gu W, Yan YH, Pei XY, Zhang C, Ding C, Xian Y (2017) Fluorescent black phosphorus quantum dots as label-free sensing probes for evaluation of acetylcholinesterase activity. Sensors Actuators B Chem 250:601–607
Yang MD, Zhou HP, Zhang YY, Hu Z, Niu N, Yu C (2018) Controlled synthesis of polydopamine: a new strategy for highly sensitive fluorescence turn-on detection of acetylcholinesterase activity. Microchim Acta 185:132–139
Yan X, Li HX, Han XS, Su X (2015) A ratiometric fluorescent quantum dots based biosensor for organophosphorus pesticides detection by inner-filter effect. Biosens Bioelectron 74:277–283
Cancar HD, Soylemez S, Akpinar Y et al (2016) A novel acetylcholinesterase biosensor: core-shell magnetic nanoparticles incorporating a conjugated polymer for the detection of organophosphorus pesticides. ACS Appl Mater Inter 8:8058–8067
Du D, Wang J, Wang LM et al (2012) Integrated lateral flow test strip with electrochemical sensor for quantification of phosphorylated cholinesterase: biomarker of exposure to organophosphorus agents. Anal Chem 84:1380–1385
Li H, He X, Kang Z, Huang H, Liu Y, Liu J, Lian S, Tsang CHA, Yang X, Lee ST (2010) Water-soluble fluorescent carbon quantum dots and Photocatalyst design. Angewandte Chemie 122:4532–4436
Li XM, Lau SP, Tang LB, Ji R, Yang P (2013) Multicolour light emission from chlorine-doped graphene quantum dots. J Mater Chem C 44:7308–7313
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381
Li F, Yang DY, Xu HP (2019) Non-metal-heteroatom-doped carbon dots: synthesis and properties. Chem Eur J 25:1165–1176
Hu Q, Gao L, Rao S, Yang ZQ, Li T, Gong X (2019) Nitrogen and chlorine dual-doped carbon nanodots for determination of curcumin in food matrix via inner filter effect. Food Chem 280:195–202
Wang WJ, Peng JW, Li FM, Su B, Chen X, Chen X (2019) Phosphorus and chlorine co-doped carbon dots with strong photoluminescence as a fluorescent probe for ferric ions. Microchim Acta 186:32
Miao Y, He N, Zhu JJ (2010) History and new developments of assays for cholinesterase activity and inhibition. Chem Rev 110:5216–5234
Li H, Yan X, Lu G et al (2017) Carbon dot-based bioplatform for dual colorimetric and fluorometric sensing of organophosphate pesticides. Sensors Actuators B Chem 260:563–570
Zhong Y, Chen Q, Li JJ et al (2017) One-step synthesis of nitrogen and chlorine co-doped carbon quantum dots for detection of Fe3+. Nano 12:17501351–17501359
Xiao SJ, Chu ZJ, Zhao XJ, Zhang ZB, Liu YH (2017) Off-on-off detection of the activity of acetylcholine esterase and its inhibitors using MoOx quantum dots as a photoluminescent probe. Microchim Acta 184:4853–4860
Zhang YY, Zhang CY, Chen J, Li Y, Yang M, Zhou H, Shahzad SA, Qi H, Yu C, Jiang S (2017) A real-time fluorescence turn-on assay for acetylcholinesterase activity based on the controlled release of a perylene probe from MnO2 nanosheets. J Mater Chem C 5:4691–4694
Qian ZS, Chai LJ, Tang C, Huang Y, Chen J, Feng H (2016) A fluorometric assay for acetylcholinesterase activity and inhibitor screening with carbon quantum dots. Sensor Actuat B-Chem 222:879–886
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (21707030) and Wuhan Youth Science and technology plan (2016070204010133).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 3694 kb)
Rights and permissions
About this article
Cite this article
Yang, M., Liu, M., Wu, Z. et al. Carbon dots co-doped with nitrogen and chlorine for “off-on” fluorometric determination of the activity of acetylcholinesterase and for quantification of organophosphate pesticides. Microchim Acta 186, 585 (2019). https://doi.org/10.1007/s00604-019-3715-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3715-z