Abstract
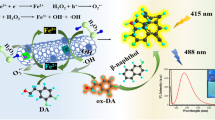
A bioinspired fluorometric method has been developed for the detection of glutathione (GSH) in biological fluids. It is based on the use of near-infrared fluorescent semiconducting polymer dots (P-dots) and of the dopamine (DA)-melanin nanosystem. The P-dots were prepared from poly(styrene-co-maleic anhydride), the semiconducting polymer poly[(9,9′-dioctyl-2,7-divinylenefluorenylene)-alt-2-methoxy-5-(2-ethyl-hexyloxy)-1,4-phenylene] and the fluorescent dye tetraphenylporphyrin. They have excitation/emission maxima at 458/656 nm, and this enables measurement to be performed with low autofluorescence and scattering background. DA can self-polymerize on the surface of the P-dots to yield a poly-DA coating. This coating, at weak alkaline pH values, causes the quenching of the fluorescence of the P-dots. However, the polymerization of DA is inhibited by GSH. Hence, quenching of fluorescence is prevented. This effect was used to design a fluorometric assay for GSH that has good selectivity and sensitivity. Under optimal conditions, the method has a linear response in the 0.2 to 20 μM GSH concentration range and a 60 nM detection limit. It was successfully applied to the determination of GSH in HepG2 cells and in spiked human serum.

Schematic representation of using a NIR fluorescent P-dots and dopamine (DA)-melanin nanohybrid as a probe for glutathione (GSH) detection. The P-dots were prepared from poly(styrene-co-maleic anhydride) (PSMA), the semiconducting polymer poly[(9,9′-dioctyl-2,7-divinylenefluorenylene)-alt-2-methoxy-5-(2-ethyl-hexyloxy)-1,4-phenylene] (PEPV) and the fluorescent dye tetraphenylporphyrin (TPP).The GSH can inhibit the dopamine self-polymerization and prevented the formation of PDA and fluorescence quenching of P-dots.






Similar content being viewed by others
References
Valko M, Rhodes CJ, Moncol J et al (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40. https://doi.org/10.1016/j.cbi.2005.12.009
Pastore A, Federici G, Bertini E, Piemonte F (2003) Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 333:19–39. https://doi.org/10.1016/S0009-8981(03)00200-6
Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921. https://doi.org/10.1016/S0891-5849(99)00177-X
Dröge W, Breitkreutz R (2000) Glutathione and immune function. Proc Nutr Soc 59:595–600. https://doi.org/10.1017/S0029665100000847
Estrela JM, Ortega A, Obrador E (2006) Glutathione in Cancer Biology and Therapy. Crit Rev Clin Lab Sci 43:143–181. https://doi.org/10.1080/10408360500523878
Ballatori N, Krance SM, Notenboom S et al (2009) Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 390:191–214. https://doi.org/10.1515/BC.2009.033
Wu G, Fang Y-Z, Yang S et al (2004) Glutathione Metabolism and Its Implications for Health. J Nutr 134:489–492. https://doi.org/10.1093/jn/134.3.489
McDermott GP, Francis PS, Holt KJ et al (2011) Determination of intracellular glutathione and glutathione disulfide using high performance liquid chromatography with acidic potassium permanganate chemiluminescence detection. Analyst 136:2578–2585. https://doi.org/10.1039/c1an00004g
Lee PT, Goncalves LM, Compton RG (2015) Electrochemical determination of free and total glutathione in human saliva samples. Sensors Actuators B Chem 221:962–968. https://doi.org/10.1016/j.snb.2015.07.050
Feng J, Huang P, Shi S et al (2017) Colorimetric detection of glutathione in cells based on peroxidase-like activity of gold nanoclusters: A promising powerful tool for identifying cancer cells. Anal Chim Acta 967:64–69. https://doi.org/10.1016/j.aca.2017.02.025
Liu J, Meng L, Fei Z et al (2017) MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens Bioelectron 90:69–74. https://doi.org/10.1016/j.bios.2016.11.046
Xu H-H, Deng H-H, Lin X-Q et al (2017) Colorimetric glutathione assay based on the peroxidase-like activity of a nanocomposite consisting of platinum nanoparticles and graphene oxide. Microchim Acta 184:3945–3951. https://doi.org/10.1007/s00604-017-2429-3
Gao W, Liu Z, Qi L et al (2016) Ultrasensitive Glutathione Detection Based on Lucigenin Cathodic Electrochemiluminescence in the Presence of MnO2 Nanosheets. Anal Chem 88:7654–7659. https://doi.org/10.1021/acs.analchem.6b01491
Wei C, Liu X, Gao Y et al (2018) Thiol–Disulfide Exchange Reaction for Cellular Glutathione Detection with Surface-Enhanced Raman Scattering. Anal Chem 90:11333–11339. https://doi.org/10.1021/acs.analchem.8b01974
Zhang X-L, Zheng C, Guo S-S et al (2014) Turn-On Fluorescence Sensor for Intracellular Imaging of Glutathione Using g-C3N4 Nanosheet–MnO2 Sandwich Nanocomposite. Anal Chem 86:3426–3434. https://doi.org/10.1021/ac500336f
Zhang D, Zheng A, Li J, et al (2017) Tumor microenvironment activable self-assembled DNA hybrids for pH and redox dual-responsive chemotherapy/PDT treatment of hepatocellular carcinoma. Adv Sci 1600460. https://doi.org/10.1002/advs.201600460
Yang Y, Zhao Q, Feng W, Li F (2013) Luminescent Chemodosimeters for Bioimaging. Chem Rev 113:192–270. https://doi.org/10.1021/cr2004103
Niu L-Y, Guan Y-S, Chen Y-Z et al (2012) BODIPY-Based Ratiometric Fluorescent Sensor for Highly Selective Detection of Glutathione over Cysteine and Homocysteine. J Am Chem Soc 134:18928–18931. https://doi.org/10.1021/ja309079f
Liu J, Bao C, Zhong X et al (2010) Highly selective detection of glutathione using a quantum-dot-based OFF-ON fluorescent probe. Chem Commun (Camb) 46:2971–2973. https://doi.org/10.1039/b924299f
Tian D, Qian Z, Xia Y, Zhu C (2012) Gold Nanocluster-Based Fluorescent Probes for Near-Infrared and Turn-On Sensing of Glutathione in Living Cells. Langmuir 28:3945–3951. https://doi.org/10.1021/la204380a
Zhou L, Lin Y, Huang Z et al (2012) Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem Commun 48:1147–1149. https://doi.org/10.1039/C2CC16791C
Deng R, Xie X, Vendrell M et al (2011) Intracellular Glutathione Detection Using MnO2 -Nanosheet-Modified Upconversion Nanoparticles. J Am Chem Soc 133:20168–20171. https://doi.org/10.1021/ja2100774
Feng L, Zhu C, Yuan H et al (2013) Conjugated polymer nanoparticles: preparation, properties, functionalization and biological applications. Chem Soc Rev 42:6620–6633. https://doi.org/10.1039/c3cs60036j
Wu C, Chiu DT (2013) Highly Fluorescent Semiconducting Polymer Dots for Biology and Medicine. Angew Chem Int Ed 52:3086–3109. https://doi.org/10.1002/anie.201205133
Lyu Y, Pu K (2017) Recent Advances of Activatable Molecular Probes Based on Semiconducting Polymer Nanoparticles in Sensing and Imaging. Adv Sci 4:1600481. https://doi.org/10.1002/advs.201600481
Guo L, Niu G, Zheng X et al (2017) Single Near-Infrared Emissive Polymer Nanoparticles as Versatile Phototheranostics. Adv Sci 4:1700085. https://doi.org/10.1002/advs.201700085
Yin C, Zhen X, Fan Q et al (2017) Degradable Semiconducting Oligomer Amphiphile for Ratiometric Photoacoustic Imaging of Hypochlorite. ACS Nano 11:4174–4182. https://doi.org/10.1021/acsnano.7b01092
Sun K, Tang Y, Li Q et al (2016) In Vivo Dynamic Monitoring of Small Molecules with Implantable Polymer-Dot Transducer. ACS Nano 10:6769–6781. https://doi.org/10.1021/acsnano.6b02386
Wang D, Chen C, Ke X et al (2015) Bioinspired Near-Infrared-Excited Sensing Platform for in Vitro Antioxidant Capacity Assay Based on Upconversion Nanoparticles and a Dopamine–Melanin Hybrid System. ACS Appl Mater Interfaces 7:3030–3040. https://doi.org/10.1021/am5086269
Matsuki M, Watanabe T, Ogasawara A et al (2008) Inhibitory Mechanism of Melanin Synthesis by Glutathione. Yakugaku Zasshi 128:1203–1207. https://doi.org/10.1248/yakushi.128.1203
Ahsan H (2011) Arsenic in drinking water. Kaohsiung J Med Sci 27:358–359. https://doi.org/10.1016/j.kjms.2011.05.002
Saha A, Jana NR (2013) Detection of Cellular Glutathione and Oxidized Glutathione Using Magnetic–Plasmonic Nanocomposite-Based “Turn-Off” Surface Enhanced Raman Scattering. Anal Chem 85:9221–9228. https://doi.org/10.1021/ac4019457
Yu F, Li P, Wang B, Han K (2013) Reversible Near-Infrared Fluorescent Probe Introducing Tellurium to Mimetic Glutathione Peroxidase for Monitoring the Redox Cycles between Peroxynitrite and Glutathione in Vivo. J Am Chem Soc 135:7674–7680. https://doi.org/10.1021/ja401360a
Wang Y, Jiang K, Zhu J et al (2015) A FRET-based carbon dot–MnO2 nanosheet architecture for glutathione sensing in human whole blood samples. Chem Commun 51:12748–12751. https://doi.org/10.1039/C5CC04905A
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21605021 and 21705022), Joint Funds for the Innovation of Science and Technology of Fujian province (Grant No. 2016Y9060 and 2017Y9115), the Young and Middle-aged Talent Training Project of Fujian Provincial Health and Family Planning Commission (Grant No. 2018-ZQN-75), the China Postdoctoral Science Foundation, the Natural Science Foundation of Fujian Province of China (Grant No. 2016 J05206), the Medical Innovation grant of Fujian province (Grant No. 2018-CX-49) and the Startup Fund of Mengchao Hepatobiliary Hospital of Fujian Medical University (Grant No. QDZJ-2017-004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 845 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Zheng, C., Tan, X. et al. Sensitive fluorometric determination of glutathione using fluorescent polymer dots and the dopamine-melanin nanosystem. Microchim Acta 186, 568 (2019). https://doi.org/10.1007/s00604-019-3675-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3675-3