Abstract
A sandwich–type electrochemical aptasensor has been constructed and applied for sensitive and selective detection of the carcinoembryonic antigen (CEA). The surface of a glassy carbon electrode (GCE) was first modified with nitrogen–doped graphene and then gold nanoparticles and graphene quantum dots electrodeposited on it to obtain an architecture of type GQD/AuNP/NG/GCE. In the next step, the CEA-binding aptamer was immobilized on the modified GCE. Hemin intercalates in the amino-modified hemin aptamer to form a hemin–G–quadruplex (hemin–G4) DNAzyme. The amino modified CEA aptamer II is connected to hemin–G4 by glutaraldehyde (GA) as a linker to produce CEAaptamerII/GA/hemin–G4 (=ApII/GA/DNAzyme). Through a sandwich mode, the ApII/GA/DNAzyme bioconjugates are captured on the modified GCE. Subsequently, the hemin–G4 acts as peroxidase-mimicking DNAzyme and rapidly catalyzes the electroreduction of hydrogen peroxide. The quantitative determination of CEA was achieved by differential pulse voltammetry, best at a working potential of around −0.27 V vs. Ag/AgCl. Under optimized conditions, the assay has a linear response in the 10.0 fg mL−1 to 200.0 ng mL−1 CEA concentration range and a lower detection limit of 3.2 fg mL−1.

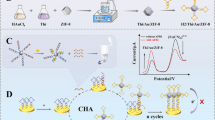
Schematic presentation of a sandwich–type electrochemical aptasensor based on nitrogen doped graphene (NG), gold nanoparticles (AuNPs) and graphene quantum dots (GQDs) modified glassy carbon electrode, and the hemin–G4 DNAzyme for femtomolar detection of the carcinoembryonic antigen.





Similar content being viewed by others
References
Xu S, Dong B, Zhou DL, Yin Z, Cui SB, Xu W, Chen BJ, Song HW (2016) Paper–based upconversion fluorescence resonance energy transfer biosensor for sensitive detection of multiple cancer biomarkers. Sci Rep 6:1–9
Longo G (2014) Cancer biomarkers: detected twice for good measure. Nat Nanotechnol 9:959–960
Qureshi A, Gurbuz Y, Niazi JH (2015) Capacitive aptamer–antibody based sandwich assay for the detection of VEGF cancer biomarker in serum. Sens Actuator B Chem 209:645–651
Iliuk AB, Hu L, Tao WA (2011) Aptamer in bioanalytical applications. Anal Chem 83:4440–4452
Ahour F, Ahsani MK (2016) An electrochemical label–free and sensitive thrombin aptasensor based on graphene oxide modified pencil graphite electrode. Biosens Bioelectron 86:764–769
Wu B, Jiang R, Wang Q, Huang J, Yang X, Wang K, Li W, Chen N, Li Q (2016) Detection of C–reactive protein using nanoparticle–enhanced surface plasmon resonance using an aptamer–antibody sandwich assay. Chem Commun 52:3568–3571
Yang JM, Dou BT, Yuan R, Xiang Y (2016) Proximity binding and metal ion–dependent DNAzyme cyclic amplification–integrated aptasensor for label–free and sensitive electrochemical detection of thrombin. Anal Chem 88:8218–8823
Palchetti I, Mascini M (2012) Electrochemical nanomaterial–based nucleic acid aptasensors. Anal Bioanal Chem 402:3103–3114
Ensafi AA, Nabiyan A, Jafari-Asl M, Dinari M, Farrokhpour H, Rezaei B (2016) Galvanic exchange at layered doubled hydroxide/N-doped graphene as an in-situ method to fabricate powerful electrocatalysts for hydrogen evolution reaction. Energy 116:1087–1096
Suresh Balaji S, Elavarasan A, Sathish M (2016) High performance supercapacitor using N-doped graphene prepared via supercritical fluid processing with an oxime nitrogen source. Electrochim Acta 200:37–45
Suhag D, Kumar Sharma A, Patni P, Kumar Garg S, Rajput SK, Chakrabarti S, Mukherjee M (2016) Hydrothermally functionalized biocompatible nitrogen doped graphene nanosheets based biomimetic platforms for nitric oxide detection. J Mater Chem B 4:4780–4789
Yang G, Li L, Kumar Rana R, Zhu JJ (2013) Assembled gold nanoparticles on nitrogen-doped graphene for ultrasensitive electrochemical detection of matrix metalloproteinase-2. Carbon 61:357–366
Tertis M, Ciui B, Suciu M, Suandulescu R, Cristea C (2017) Label-free electrochemical aptasensor based on gold and polypyrrole nanoparticles for interleukin 6 detection. Electrochim Acta 258:1208–1218
Zhao J, Chen G, Zhu L, Li G (2011) Graphene quantum dots-based platform for the fabrication of electrochemical biosensors. Electrochem Commun 13:31–33
Xie R, Wang Z, Zhou W, Liu Y, Fan L, Li Y, Li X (2016) Graphene quantum dots as a smart probe for biosensing. Anal Methods 8:4001–4016
Li Y, Deng J, Fang L, Yu K, Huang H, Jiang L, Liang W, Zheng J (2015) A novel electrochemical DNA biosensor based on HRP-mimicking hemin/G-quadruplex wrapped GOx nanocomposites as tag for detection of Escherichia coli O157:H7. Biosens Bioelectron 63:1–6
Xie S, Chai Y, Yuan R, Bai L, Yuan Y, Wang Y (2012) A dual-amplification aptasensor for highly sensitive detection of thrombin based on the functionalized graphene-Pd nanoparticles composites and the hemin/G-quadruplex. Anal Chim Acta 755:46–53
Shekari Z, Zare HR, Falahati A (2017) An ultrasensitive aptasensor for hemin and hemoglobin based on signal amplification via electrocatalytic oxygen reduction. Anal Biochem 518:102–109
Wang GL, Shu JX, Dong YM, Wu XM, Zhao WW, Xu JJ, Chen HY (2015) Using G–quadruplex/hemin to "switch–on" the cathodic photocurrent of p–type PbS quantum dots: toward a versatile platform for photoelectrochemical aptasensing. Anal Chem 87:2892–2900
Zheng YN, Chai YQ, Yuan YL, Yuan R (2014) A pseudo triple–enzyme electrochemical aptasensor based on the amplification of Pt–Pd nanowires and hemin/G–quadruplex. Anal Chim Acta 834:45–50
Piovesan JV, Santana ER, Spinelli (2018) A reduced graphene oxide/gold nanoparticles nanocompositemodified glassy carbon electrode for determination of endocrine disruptor methylparaben. J Electroanal Chem 813:163–170
Song J, Kim CM, Yang E, Ham MH, Kim IS (2017) The effect of doping temperature on the nitrogenbonding configuration of nitrogen-doped graphene by hydrothermal treatment. RSC Adv 7:20738–20741
Akbarnia A, Zare HR (2018) A voltammetric assay for microRNA-25 based on the use of amino-functionalized graphene quantum dots and ss- and ds-DNAs as gene probes. Microchim Acta 185(11):503–510
Yang JJ, Cao JT, Wang YL, Wang H, Liua YM, Ma SH (2017) Sandwich-like electrochemiluminescence aptasensor based on dual quenching effect from hemin-graphene nanosheet and enzymatic biocatalytic precipitation for sensitive detection of carcinoembryonic antigen. J Electroanal Chem 787:88–94
Bard AJ, Faulkner LR (2001) Electrochemical methods. In: Fundamentals and applications. Wiley, New York
Miller JN, Miller JC (2000) Statistics and Chemometrics for analytical chemistry. 4th ed, Pearson Education, UK
Shekari Z, Zare HR, Falahati A (2017) Developing an impedimetric aptasensor for selective label–free detection of CEA as a cancer biomarker based on gold nanoparticles loaded in functionalized mesoporous silica films. J Electrochem Soc 164:B739–B745
He Y, Chai YQ, Wang HJ, Bai LJ, Yuan R (2014) A signal–on electrochemiluminescence aptasensor based on the quenching effect of manganese dioxide for sensitive detection of carcinoembryonic antigen. RSC Adv 4:56756–56761
Si Z, Xie B, Chen Z, Tang C, Li T, Yang M (2017) Electrochemical aptasensor for the cancer biomarker CEA based on aptamer induced current due to formation of molybdophosphate. Microchim Acta 184:3215–3221
Gao J, Guo ZK, Su FJ, Gao L, Pang XH, Cao W, Du B, Wei Q (2015) Ultrasensitive electrochemical immunoassay for CEA through host–guest interaction of beta–cyclodextrin functionalized graphene and cu@ag core–shell nanoparticles with adamantine–modified antibody. Biosens Bioelectron 63:465–471
Taghdisi SM, Danesh NM, Ramezani M, Sarreshtehdar Emrani A, Abnous K (2018) A novel electrochemical aptasensor for carcinoembryonic antigen detection based on target-induced bridge assembly. Electroanalysis 30:1–7
Wang P, Wan Y, Su Y, Deng S, Yang S (2016) Ultrasensitive electrochemical aptasensor based on surface-initiated enzymatic polymerization. Chin J Chem 34:337–341
Wildt RMT, Mundy CR, Gorick BD, Tomlinson M (2000) Antibody arrays for high-throughput screening of antibody–antigen interactions. Nat Biotechnol 18:989–994
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 328 kb)
Rights and permissions
About this article
Cite this article
Shekari, Z., Zare, H.R. & Falahati, A. Electrochemical sandwich aptasensor for the carcinoembryonic antigen using graphene quantum dots, gold nanoparticles and nitrogen doped graphene modified electrode and exploiting the peroxidase-mimicking activity of a G-quadruplex DNAzyme. Microchim Acta 186, 530 (2019). https://doi.org/10.1007/s00604-019-3572-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3572-9