Abstract
The authors describe an electrochemical immunoassay for simultaneous determination of alpha-fetoprotein (AFP) and glypican-3 (GPC-3) which are important biomarkers for early detection of hepatocellular carcinoma (HCC). Magnetite (Fe3O4) nanoparticles (NPs) were decorated with hyperbranched amino functionalized dendrimers. The modified NPs were coupled to the antibodies against AFP and GPC-3. The electrochemical behaviour of the Fe3O4 NPs and dendrimer-modified NPs were studied. A glassy carbon electrode was then modified with the NP-conjugated antibodies and biomolecular interactions were studied by using cyclic voltammetry and electrochemical impedance spectroscopy. Dual differential pulse voltammetric sensing was performed by utilizing the redox probes; Prussian blue for AFP and toluidine blue for GPC-3. The biomarkers can be detected best at voltages of 0.25 mV and – 0.54 mV (vs Ag/AgCl) for AFP and GPC-3, respectively. The low working potentials makes the method more selective over other electroactive species present in real human serum samples. Response is linear in 0.02 to 10 ng mL−1 concentration ranges of both AFP and GPC-3; and the respective detection limits are 50 and 70 pg mL−1. The method was validated by analysing spiked human serum samples. In our perception, the method is of great clinical significance as combination of GPC-3 and AFP increases the sensitivity of detection of HCC.

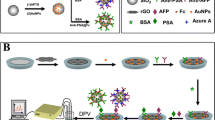
Schematic presentation of polyamidoamine (PAMAM) dendrimers modified magnetite (Fe3O4) nanoparticles as electrochemical sensing platform using redox dyes Prussian blue and toluidine blue for simultaneous detection of alpha-fetoprotein (AFP) and glypican-3 (GPC-3), respectively by differential pulse voltammetry.






Similar content being viewed by others
References
Yezaz AG, Idrees M, Julie HR (2017) Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog 16:1–17. https://doi.org/10.4103/jcar.JCar_9_16
Schraml C, Kaufmann S, Rempp H, Syha R, Ketelsen D, Notohamiprodjo M, Nikolaou K (2015) Imaging of HCC – current state of the art. Diagnostics 5:513–545. https://doi.org/10.3390/diagnostics5040513
Hina AT, Bobby K, Surya C, Puneet S, James C, Rainner WG, Diego RM (2014) MRI of hepatocellular carcinoma: an update of current practices. Diagn Interv Radiol 20:209–221. https://doi.org/10.5152/dir.2014.13370
Liu QL, Yan XH, Yin XM, Situ B, Zhou HK, Lin L, Li B, Gan N, Zheng L (2013) Electrochemical enzyme-linked immunosorbent assay (ELISA) for α-fetoprotein based on glucose detection with multienzyme-nanoparticle amplification. Molecules 18:12675–12686. https://doi.org/10.3390/molecules181012675
Sallam KM, Sheha R, El-Zahhar AA (2011) Development of solid phase radioimmunoassay system using new polymeric magnetic micro-spheres. J Radioanal Nucl Chem 290:339–345. https://doi.org/10.1007/s10967-011-1188-6
Limei A, Feng G, Bifeng P, Rong H, Daxiang C (2006) Fluoroimmunoassay for antigen based on florescence quenching signal of gold nanoparticles. Anal Chem 78:1104–1106. https://doi.org/10.1021/ac051323m
Sandeep KV, Venkatesh AG, Konstantinos M, Gregor C, Gunter R, Felix von S, Roland Z (2012) Nanotechnology-based biosensors and diagnostics: technology push versus industrial/healthcare requirements. BioNanoScience 2:115–126. https://doi.org/10.1007/s12668-012-0047-4
Sudeshna C, Mumukshu DP, Heinrich L, Dhirendra B (2015) Dendrimer-functionalized magnetic nanoparticles: a new electrode material for electrochemical energy storage devices. J Power Sources 280:217–226. https://doi.org/10.1016/j.jpowsour.2015.01.075
Rouhollah K, Gozde U, Serap Y, Gungor G, Ufuk G (2013) PAMAM dendrimer-coated iron oxide nanoparticles: synthesis and characterization of different generations. J Nanopart Res 15:1488–1486. https://doi.org/10.1007/s11051-013-1488-6
Vasile E, Serafim A, Petre D, Giol D, Dubruel P, Iovu H, Stancu I (2014) Direct synthesis and morphological characterization of gold-dendrimer nanocomposites prepared using PAMAM succinamic acid dendrimers: preliminary study of the calcification potential. Sci World J 918:1–15. https://doi.org/10.1155/2014/103462
Yan-Jie Z, Qiang J, Guan-Cheng L (2013) Tumor markers for hepatocellular carcinoma. Molecular and Clinical Oncology 1:593–598. https://doi.org/10.3892/mco.2013.119
Hanlin W, Florencia A, Qihui Z, Brian A, Shang-Tian C, Ximing Y (2008) Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med 132:1723–1728. https://doi.org/10.1043/1543-2165-132.11.1723
Tangkijvanich P, Chanmee T, Komtong S, Mahachai V, Wisedopas N, Pothacharoen P, Kongtawelert P (2010) Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J Gastroenterol Hepatol 25:129–137. https://doi.org/10.1111/j.1440-1746.2009.05988.x
Jiatao L, Lingfei Z, Shaogang LV, Chenzi Z, Shuai J (2017) Biomarkers for hepatocellular carcinoma. Biomark Cancer 9:1–9. https://doi.org/10.1177/1179299X16684640
Sascha D, Sudeshna C, Colin G, Senoy T, Denys M, Steffen S, Michael H, Manfred A, Dhirendra B, Heinrich L (2012) Design, characterization and magnetic properties of Fe3O4-nanoparticle arrays coated with PEGylated-dendrimers. Mater Chem Phys 132:292–299. https://doi.org/10.1016/j.matchemphys.2011.11.015
Milton C, Fabio FC, Pedro P, Antonio L, Pedro VB (2016) Gold nanoparticles for diagnostics: advances towards points of care. Diagnostics 6:1–20. https://doi.org/10.3390/diagnostics6040043
Guifen J, Lei W, Jinxin Y, Shusheng Z (2011) Versatile electrochemiluminescence assays for cancer cells based on dendrimer/CdSe-ZnS-quantum dot nanoclusters. Anal Chem 83:3873–3880. https://doi.org/10.1021/ac200383z
Joseph W (2005) Carbon-nanotube based electrochemical biosensors: a review. Electroanalysis 17:7–14. https://doi.org/10.1002/elan.200403113
Ming Z, Yueming Z, Shaojun D (2009) Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal Chem 81:5603–5613. https://doi.org/10.1021/ac900136z
Ashok KS, Shen-Ming C (2008) Nanostructured zinc oxide particles in chemically modified electrodes for biosensor applications. Anal Lett 41:141–158. https://doi.org/10.1080/00032710701792612
Ni Y, Zhu J, Zhang L, Hong J (2010) Hierarchical ZnO micro/nanoarchitectures: hydrothermal preparation, characterization and application in the detection of hydrazine. CrystEngComm 12:2213–2218. https://doi.org/10.1039/b923857n
Yu Y, Cuiwen J, Tao L, Jie L, Yegeng F, Yuning W, Feiyan Y, Liping X (2017) A zinc oxide nanoflower-based electrochemical sensor for trace detection of sunset yellow. Sensors 17:1–9. https://doi.org/10.3390/s17030545
Kim ES, Thomas P, Igor LM, Hedi M (2006) Biosensing with luminescent semiconductor quantum dots. Sensors 6:925–953. https://doi.org/10.3390/s6080925
Cho IH, Lee J, Kim J, Kang MS, Paik JK, Ku S, Cho HM, Irudayaraj J, Kim DH (2018) Current technologies of electrochemical immunosensors: perspective on signal amplification. Sensors 18:1–18. https://doi.org/10.3390/s18010207
Almira R, Arunas R (2002) Application of polypyrrole for the creation of immunosensors. Crit Rev Anal Chem 32:245–252. https://doi.org/10.1080/10408340290765542
Ling D, Lee N, Hyeon T (2015) Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications. Acc Chem Res 48:1276–1285. https://doi.org/10.1021/acs.accounts.5b00038
Michael H, Alan LG, Serge C (2014) Nanomaterials for biosensing applications: a review. Frontiers in Chemistry 2:1–10. https://doi.org/10.3389/fchem.2014.00063
Li G, Li S, Wang Z, Xue Y, Dong C, Zeng J, Huang Y, Liang J, Zhou Z (2018) Label-free electrochemical aptasensor for detection of alpha-fetoprotein based on AFP-aptamer and thionin/reduced graphene oxide/gold nanoparticles. Anal Biochem 547:37–44. https://doi.org/10.1016/j.ab.2018.02.012
Li S, Liang J, Zhou Z, Li G (2018) An electrochemical immunosensor for AFP measurement based on the magnetic Fe3O4@au@CS nanomaterials. Mater Sci Eng 382:1–6. https://doi.org/10.1088/1757-899X/382/2/022017
Yuan Y, Li S, Xue Y, Liang J, Cui L, Li Q, Zhou S, Huang Y, Li G, Zhao Y (2017) A Fe3O4@au-based pseudo-homogeneous electrochemical immunosensor for AFP measurement using AFP antibody-GNPs-HRP as detection probe. Anal Biochem 534:56–63. https://doi.org/10.1016/j.ab.2017.07.015
Tingting X, Bo C, Jian G, Meilin C, Wenlu F, Meihui Y, Hong X (2017) Novel electrochemical immune sensor based on Hep-PGA-PPy nanoparticles for detection of α-fetoprotein in whole blood. Analytica Chimica Acta 977:36–43. https://doi.org/10.1016/j.aca.2017.04.045
Xu T, Chi B, Wu F, Ma S, Zhan S, Yi M, Xu H, Mao C (2017) A sensitive label-free immunosensor for detection α-fetoprotein in whole blood based on anticoagulating magnetic nanoparticles. Biosens Bioelectron 95:87–93. https://doi.org/10.1016/j.bios.2017.04.015
Huang Z-J, Han W-D, Wu Y-H, Hu X-G (2016) Magnetic electrochemiluminescent immunoassay with quantum dots label for highly efficient detection of the tumor marker α-fetoprotein. J Electroanal Chem 785:8–13. https://doi.org/10.1016/j.jelechem.2016.11.060
Lin J, Zhang H, Niu S (2015) Simultaneous determination of carcinoembryonic antigen and α-fetoprotein using an ITO immunoelectrode modified with gold nanoparticles and mesoporous silica. Microchim Acta 182:719–726. https://doi.org/10.1007/s00604-014-1378-3
Acknowledgments
The authors are thankful to Department of Metallurgical Engineering and Materials Science, Indian Institute of Technology Bombay, for XRD and VSM measurements. We acknowledge Dr. Kolja Them (University of Hamburg, Germany) for performing nanoparticle characterization by TEM. Authors are grateful for financial support from Nanomission, Department of Science and Technology [Sanction No.: SR/NM/NB-1024/2016(C)], Government of India for providing research project grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval was obtained from Institutional Ethics Committee (NMIMS/IEC/010/2018).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.91 mb)
Rights and permissions
About this article
Cite this article
Chikhaliwala, P., Rai, R. & Chandra, S. Simultaneous voltammetric immunodetection of alpha-fetoprotein and glypican-3 using a glassy carbon electrode modified with magnetite-conjugated dendrimers. Microchim Acta 186, 255 (2019). https://doi.org/10.1007/s00604-019-3354-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3354-4