Abstract
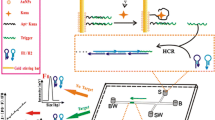
A simple and highly sensitive fluorometric method is described for the determination of the antibiotic kanamycin (Kana) in food. Dual signal amplification is accomplished by making use of double Y-shaped aptamer DNA probes acting as a capture probes and signal amplification probes. The DNA probes were immobilized on a gold bar and on a magnetic bar, respectively. On addition of Kana, the Y-shaped aptamer probe captures Kana and then is disassembled to release two single-stranded DNAs. These trigger target recycling and HCR between the two bars simultaneously. As a result, many long duplex DNA chains are formed in the supernatant. After pulling out the bars and adding the fluorescent intercalating probe SYBR Green I, strong fluorescence (with excitation/emission peaks at 497/525 nm) is induced. The use of such double Y-shaped DNA probes obviously overcomes the unspecific signal amplification by HCR which increases selectivity and sensitivity. This is due to the fact that the hairpin of HCR is separated in being present in different arms of the Y-shaped probe. Under the optimal conditions, the assay has a limit of 0.45 pg·mL−1 for Kana. It was applied to analyze spiked milk, fish and pork samples.

The scheme represents a sensitive fluorometric aptamer-based method to detect kanamycin (Kana). It is making use of a double stirring bar-assisted dual amplification strategy with zero background. Abbreviations: apt: aptamer, AuNPs: gold nanoparticles, HCR: hybridization chain reaction.





Similar content being viewed by others
References
Robati RY, Arab A, Ramezani M, Langroodi FA, Abnous K, Taghdisi SM (2016) Aptasensors for quantitative detection of kanamycin. Biosens Bioelectron 82:162–172
Economou V, Gousia P (2015) Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infection and Drug Resistance 8:49–61
Oertel R, Neumeister V, Kirch W (2004) Hydrophilic interaction chromatography combined with tandem-mass spectrometry to determine six aminoglycosides in serum. J Chromatogr A 1058(1–2):197–201
Wang C, Chen D, Wang Q, Tan R (2016) Kanamycin detection based on the catalytic ability enhancement of gold nanoparticles. Biosens Bioelectron 91:262–267. https://doi.org/10.1016/j.bios.2016.12.042
Lin YF, Wang YC, Chang SY (2008) Capillary electrophoresis of aminoglycosides with argon-ion laser-induced fluorescence detection. J Chromatogr A 1188(2):331–333
Patel KN, Limgavkar RS, Raval HG, Patel KG, Gandhi TR (2015) High-performance liquid chromatographic determination of Cefalexin monohydrate and kanamycin Monosulfate with Precolumn derivatization. J Liq Chromatogr Relat Technol 38(6):716–721
Yu S, Wei Q, Du B, Wu D, Li H, Yan L, Ma H, Zhang Y (2013) Label-free immunosensor for the detection of kanamycin using ag@Fe3O4 nanoparticles and thionine mixed graphene sheet. Biosens Bioelectron 48(19):224–229
Wu S, Wang Y, Duan N, Ma H, Wang Z (2015) Colorimetric Aptasensor based on enzyme for the detection of Vibrio parahemolyticus. J Agric Food Chem 63(35):7849–7854
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822. https://doi.org/10.1038/346818a0
Sun Y, Ren Q, Liu B, Qin Y, Zhao S (2016) Enzyme-free and sensitive electrochemical determination of the FLT3 gene based on a dual signal amplified strategy: controlled nanomaterial multilayers and a target-catalyzed hairpin assembly. Biosens Bioelectron 78:7–13
Yang D, Tang Y, Miao P (2017) Hybridization chain reaction directed DNA superstructures assembly for biosensing applications. TrAC Trends Anal Chem 94:1–3
Liu P, Yang X, Sun S, Wang Q, Wang K, Huang J, Liu J, He L (2013) Enzyme-free colorimetric detection of DNA by using gold nanoparticles and hybridization chain reaction amplification. Anal Chem 85(16):7689–7695
Lv X, Wu W, Niu C, Huang D, Wang X, Zhang X (2016) A facile "turn-on" fluorescent method with high sensitivity for Hg(2+) detection using magnetic Fe3O4 nanoparticles and hybridization chain reactions. Talanta 151:62–67. https://doi.org/10.1016/j.talanta.2016.01.025
Ning S, Ke W, Wang L, Xie F, Li T, Qiang B, Di Z, Liu M, Yu WW (2017) Ultrasensitive detection of hg (II) through metal-enhanced fluorescence and hybridization chain reaction. Sensors Actuators B Chem 245:568–573
Ying C, Ling C, Ou Y, Wang Z, Fu F, Guo L (2016) DNAzyme-based biosensor for Cu2+ ion by combining hybridization chain reaction with fluorescence resonance energy transfer technique. Talanta 155:245–249
Wang X, Jiang A, Hou T, Li H, Li F (2015) Enzyme-free and label-free fluorescence aptasensing strategy for highly sensitive detecion of protein based on target-triggered hybridization chain reaction amplification. Biosens Bioelectron 70:324–329
Z L BL, Y Z HY, J W SA (2017) Ultrasensitive microRNA-21 detection based on DNA hybridization chain reaction and SYBR green dye. Anal Biochem 538:20–25
Wang B, Zhou X, Yao D, Sun X, He M, Wang X, Yin X, Liang H (2017) Contribution of gold nanoparticles to the catalytic DNA strand displacement in leakage reduction and signal amplification. Chem Commun 53(79):10950–10953
Chen X, Briggs N, Mclain JR, Ellington AD (2013) Stacking nonenzymatic circuits for high signal gain. Proc Natl Acad Sci U S A 110(14):5386–5391
Song K-M, Cho M, Jo H, Min K, Jeon SH, Kim T, Han MS, Ku JK, Ban C (2011) Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal Biochem 415(2):175–181
Zhang K, Gan N, Hu F, Chen X, Li T, Cao J (2018) Microfluidic electrophoretic non-enzymatic kanamycin assay making use of a stirring bar functionalized with gold-labeled aptamer, of a fluorescent DNA probe, and of signal amplification via hybridization chain reaction. Microchim Acta 185(3):181. https://doi.org/10.1007/s00604-017-2635-z
Zhou L, Gan N, Hu F, Li T, Cao Y, Wu D (2018) Microchip electrophoresis array-based aptasensor for multiplex antibiotic detection using functionalized magnetic beads and polymerase chain reaction amplification. Sensors Actuators B Chem 263:568–574
Wang Y, Gan N, Zhou Y, Li T, Hu F, Cao Y, Chen Y (2017) Novel label-free and high-throughput microchip electrophoresis platform for multiplex antibiotic residues detection based on aptamer probes and target catalyzed hairpin assembly for signal amplification. Biosens Bioelectron 97:100–106
Leung KH, He HZ, Chan SH, Fu WC, Leung CH, Ma DL (2013) An oligonucleotide-based switch-on luminescent probe for the detection of kanamycin in aqueous solution. Sensors Actuators B Chem 177(1):487–492
Qin X, Yin Y, Yu H, Guo W, Pei M (2016) A novel signal amplification strategy of an electrochemical aptasensor for kanamycin, based on thionine functionalized graphene and hierarchical nanoporous PtCu. Biosens Bioelectron 77:752–758
Acknowledgments
This work was supported by National Key R&D Program of China (No.2016YFD0401502), the National Natural Science Foundation of China (No.31871825), the key project of natural science foundation of china (11832013), Natural Science Foundation of Zhejiang (LY19B050001, LY17C200007), Zhejiang Province Welfare Technology Applied Research Project (2017C330040, LGC19B070003, LGN18H300001), Zhejiang provincial Top key discipline of biology (ZS2015005), the Natural Science and Huiming Foundation of Ningbo (2016A610084, 2017A610225, 2017C50035) and K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.11 mb)
Rights and permissions
About this article
Cite this article
Zhang, K., Cao, J., Wu, Y. et al. A fluorometric aptamer method for kanamycin by applying a dual amplification strategy and using double Y-shaped DNA probes on a gold bar and on magnetite nanoparticles. Microchim Acta 186, 120 (2019). https://doi.org/10.1007/s00604-018-3207-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3207-6