Abstract
Molybdenum disulfide (MoS2) was used as an electrically conductive skeleton and functionalized with an ionic liquid and gold/silver nanorods. The resulting composite was characterized by scanning electron microscopy, transmission electron microscopy and UV–vis spectroscopy. The composites were used to modify a glassy carbon electrode (GCE) to obtain a sensor for 2,4-dichlorophenol (2,4-DCP). The results show that the oxidation power and electrocatalytic activity of the modified GCE towards 2,4-DCP are enhanced compared to a bare GCE and other modified GCEs. Response is linear in the 0.01 to 50 μM 2,4-DCP concentration range, with a 2.6 nM detection limit. The sensor is highly sensitive and long-term stable. It was successfully applied to the determination of 2,4-DCP in spiked water samples and gave satisfactory recoveries.

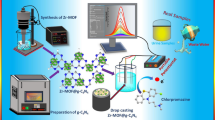
Schematic of an electrochemical sensor for the differential pulse voltammetric (DPV) determination of 2,4-dichlorophenol. It is based on the use of an MoS2-ionic liquid-Au/Ag nanorod composite.




Similar content being viewed by others
References
Mckinlay R, Plant JA, Bell JNB, Voulvoulis N (2008) Endocrine disrupting pesticides: implications for risk assessment. Environ Int 34:168–183
Chaliha S, Bhattacharyya KG, Paul P (2008) Catalytic destruction of 4-chlorophenol in water. CLEAN-Soil, Air, Water 36:488–497
Liang Y, Yu L, Yang R, Li X, Qu L, Li J (2017) High sensitive and selective graphene oxide/molecularly imprinted polymer electrochemical sensor for 2,4-dichlorophenol in water. Sensors Actuators B Chem 240:1330–1335
Gao J, Liu L, Liu X, Zhou H, Huang S, Wang Z (2008) Levels and spatial distribution of chlorophenols-2,4-dichlorophenol, 2,4,6-trichlorophenol, and pentachlorophenol in surface water of China. Chemosphere 71:1181–1187
Zheng C, Zhao J, Bao P, Gao J, He J (2011) Dispersive liquid-liquid microextraction based on solidification of floating organic droplet followed by high-performance liquid chromatography with ultraviolet detection and liquid chromatography-tandem mass spectrometry for the determination of triclosan. J Chromatogr A 1218:3830–3836
Guo L, Lee HK (2012) Electro membrane extraction followed by low-density solvent based ultrasound-assisted emulsification microextraction combined with derivatization for determining chlorophenols and analysis by gas chromatography-mass spectrometry. J Chromatogr A 1243:14–22
Huang X, Zhang Y, Mei M, Yuan D (2014) Preparation of monolithic fibers for the solid-phase microextraction of chlorophenols in water samples. J Sep Sci 37:1185–1193
Rama MJR, Medina AR, Díaz AM (2003) A simple and straightforward procedure for monitoring phenol compounds in waters by using uv solid phase transduction integrated in a continuous flow system. Microchim Acta 141:143–148
Hallaj T, Amjadi M (2016) Determination of 2,4-dichlorophenol in water samples using a chemiluminescence system consisting of graphene quantum dots, rhodamine b and cerium(iv) ion. Microchim Acta 183:1219–1225
Chen JR, Odenthal PM, Swartz AG, Floyd GC, Wen H, Luo KY, Kawakami RK (2013) Control of schottky barriers in single layer MoS2 transistors with ferromagnetic contacts. Nano Lett 13:3106–3110
Yin ZY, Li H, Li H, Jiang L, Shi YM, Sun YH, Lu G, Zhang Q, Chen XD, Zhang H (2012) Single-layer MoS2 phototransistors. ACS Nano 6:74–80
Voiry D, Yamaguchi H, Li J, Silva R, Alves DC, Fujita T, Chen M, Asefa T, Shenoy VB, Eda G, Chhowalla M (2013) Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat Mater 12:850–855
Fang LX, Huang KJ, Liu Y (2015) Novel electrochemical dual-aptamer-based sandwich biosensor using molybdenum disulfide/carbon aerogel composites and Au nanoparticles for signal amplification. Biosens Bioelectron 71:171–178
Wang GX, Bao WJ, Wang J, Lu QQ, Xia XH (2013) Immobilization and catalytic activity of horseradish peroxidase on molybdenum disulfide nanosheets modified electrode. Electrochem Commun 35:146–148
Huang KJ, Liu YJ, Wang HB, Wang YY, Liu YM (2014) Sub-femtomolar dna detection based on layered molybdenum disulfide/multi-walled carbon nanotube composites, Au nanoparticle and enzyme multiple signal amplification. Biosens Bioelectron 55:195–202
Ohno H (ed) (2005) Electrochemical aspects of ionic liquids. Wiley, Hoboken
Safavi A, Maleki N, Farjami E (2009) Fabrication of a glucose sensor based on a novel nanocomposite electrode. Biosens Bioelectron 24:1655–1660
Kachoosangi RT, Musameh MM, Abu-Yousef I, Yousef JM, Kanan SM, Xiao L, Davies SG, Russell A, Compton RG (2009) Carbon nanotube-ionic liquid composite sensors and biosensors. Anal Chem 81:435–442
Park SK, Yu SH, Woo S, Ha J, Shin J, Sung YE, Piao Y (2012) A facile and green strategy for the synthesis of MoS2 nanospheres with excellent li-ion storage properties. CrystEngComm 14:8323–8325
Zhou N, Li J, Chen H, Liao C, Chen L (2013) A functional graphene oxide-ionic liquid composites-gold nanoparticle sensing platform for ultrasensitive electrochemical detection of Hg2+. Analyst 138:1091–1097
Samal AK, Sreeprasad TS, Pradeep T (2010) Investigation of the role of NaBH4, in the chemical synthesis of gold nanorods. J Nanopart Res 12:1777–1786
Shi Y, Wang J, Wang C, Zhai TT, Bao WJ, Xu JJ, Chen HY (2015) Hot electron of Au nanorods activates the electrocatalysis of hydrogen evolution on MoS2 nanosheets. J Am Chem Soc 137:7365–7370
Zanello P (2003) Inorganic electrochemistry: theory, practice and application. The Royal Society of Chemistry, Cambridge
Brown AP, Anson FC (1977) Cyclic and differential pulse voltammetric behavior of reactants confined to the electrode surface. Anal Chem 49:1589–1595
Laviron E (1974) Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem 52:355–393
Yin H, Zhou Y, Cui L, Liu X, Ai S, Zhu L (2011) Electrochemical oxidation behavior of bisphenol A at surfactant/layered double hydroxide modified glassy carbon electrode and its determination. J Solid State Electrochem 15:167–173
Deiminiat B, Rounaghi GH, Arbab-Zavar MH, Razavipanah I (2017) A novel electrochemical aptasensor based on f-MWCNTs/AuNPs nanocomposite for label-free detection of bisphenol A. Sensors Actuators B Chem 242:158–166
Kong L, Huang S, Yue Z, Peng B, Li M, Zhang J (2009) Sensitive mediator-free tyrosinase biosensor for the determination of 2,4-dichlorophenol. Microchim Acta 165:203–209
Li J, Miao D, Yang R, Qu L, Harrington PDB (2014) Synthesis of poly(sodium 4-styrenesulfonate) functionalized graphene/cetyltrimethylammonium bromide (CTAB) nanocomposite and its application in electrochemical oxidation of 2,4-dichlorophenol. Electrochim Acta 125:1–8
Li S, Du D, Huang J, Tu H, Yang Y, Zhang A (2013) One-step electrodeposition of a molecularly imprinting chitosan/phenyltrimethoxysilane/Aunps hybrid film and its application in the selective determination of p-nitrophenol. Analyst 138:2761–2768
Li J, Li X, Yang R, Qu L, Harrington PB (2013) A sensitive electrochemical chlorophenols sensor based on nanocomposite of ZnSe quantum dots and cetyltrimethylammonium bromide. Anal Chim Acta 804:76–83
Zhu X, Zhang K, Wang C, Guan J, Yuan X, Li B (2016) Quantitative determination and toxicity evaluation of 2,4-dichlorophenol using poly(eosin Y)/hydroxylated multi-walled carbon nanotubes modified electrode. Sci Rep 6:38657
Acknowledgments
The authors gratefully acknowledge the financial support provided by the Program for Key Science and Technology Innovation Team in Shaanxi Province (No. 2014KCT-27).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 489 kb)
Rights and permissions
About this article
Cite this article
Huang, H., Wang, M., Wang, Y. et al. Electrochemical determination of 2,4-dichlorophenol by using a glassy carbon electrode modified with molybdenum disulfide, ionic liquid and gold/silver nanorods. Microchim Acta 185, 292 (2018). https://doi.org/10.1007/s00604-018-2834-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2834-2