Abstract
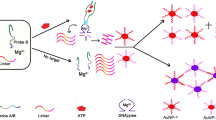
The authors describe a colorimetric method for the determination of DNA based on the deaggregation of gold nanoparticles (AuNPs) induced by exonuclease III (Exo III). DNA amplification is accomplished by Exo III to generate large quantities of the residual DNA. Residual DNA tethers onto the surfaces of AuNPs which prevents their aggregation. Hence, the color of the solution is red. However, in the absence of DNA, salt-induced aggregation is not prevented, and the bluish-purple color of the aggregated AuNPs is observed. The ratio of absorbances at 525 and 625 nm increases up to 150 nM DNA concentrations, and the LOD is as low as 3.0 nM. It is shown that the presence of 300 nM concentrations of random DNA (with a mass up to 10-fold that of target DNA) does not interfere. The method was successfully applied to the analysis of DNA in spiked serum samples. The method is simple, reliable, and does not require complicated amplification steps and expensive instrumentation.

Schematic of a sensing strategy for DNA detection by exonuclease III-induced deaggregation of gold nanoparticles. DNA concentrations as low as 3 nM can be detected via colorimetric monitoring of the color change from red to purple-blue.





Similar content being viewed by others
References
Lu W, Yuan Q, Yang Z, Yao B (2016) Self-primed isothermal amplification for genomic DNA detection of human papillomavirus. Biosens Bioelectron 90:258–263
Park BH, Oh SJ, Jung JH, Choi G, Seo JH, Kim DH, Lee EY, Seo TS (2017) An integrated rotary microfluidic system with DNA extraction, loop-mediated isothermal amplification, and lateral flow strip based detection for point-of-care pathogen diagnostics. Biosens Bioelectron 91:334–340
Zhou Q, Zheng J, Qing Z, Zheng M, Yang J, Yang S, Ying L, Yang R (2016) Detection of circulating tumor DNA in human blood via DNA-mediated surface enhanced Raman spectroscopy of single-walled carbon nanotubes. Anal Chem 88(9):4759–4765
Ji H, Yan F, Lei J, Ju H (2012) Ultrasensitive electrochemical detection of nucleic acids by template enhanced hybridization followed with rolling circle amplification. Anal Chem 84:7166–7171
Vardevanyan PO, Antonyan AP, Parsadanyan MA, Davtyan HG, Karapetyan AT (2003) The binding of ethidium bromide with DNA: interaction with single- and double-stranded structures. Exp Mol Med 35:527–533
Zhao B, Yan J, Wang D, Ge Z, He S, He D, Song S, Fan C (2013) Carbon nanotubes multifunctionalized by rolling circle amplification and their application for highly sensitive detection of cancer markers. Small 9:2595–2601
Cheng Y, Zhang X, Li Z, Jiao X, Wang Y, Zhang Y (2009) Highly sensitive determination of microRNA using target-primed and branched rolling-circle amplification. Angew Chem Int Ed 48:3268–3272
Mi L, Wen Y, Pan D, Wang Y, Fan C, Hu J (2009) Modulation of DNA polymerases with gold nanoparticles and their applications in hot-start PCR. Small 5:2597–2600
Pollet J, Janssen KP, Knez K, Lammertyn J (2011) Real-time monitoring of solid-phase PCR using fiber-optic SPR. Small 7:1003–1006
Hsieh K, Patterson AS, Ferguson BS, Plaxco KW, Soh HT (2012) Rapid, sensitive, and quantitative detection of pathogenic DNA at the point of care via microfluidic electrochemical quantitative loop-mediated isothermal amplification (MEQ-LAMP). Angew Chem Int Ed 51:4896–4900
Wong JK, Yip SP, Lee TM (2014) Ultrasensitive and closed-tube colorimetric loop-mediated isothermal amplification assay using carboxyl-modified gold nanoparticles. Small 10:1495–1499
Xue Q, Lv Y, Zhang Y, Xu S, Li R, Yue Q, Li H, Wang L, Gu X, Zhang S, Liu J (2014) Ultrasensitive fluorescence detection of nucleic acids using exonuclease III-induced cascade two-stage isothermal amplification-mediated zinc (II)-protoporphyrin IX/G-quadruplex supramolecular fluorescent nanotags. Biosens Bioelectron 61:351–356
Li JJ, Liu QY, Xi HY, Wei XC, Chen ZB (2017) Y-shaped DNA duplex structure-triggered gold nanoparticle dimers for ultrasensitive colorimetric detection of nucleic acid with the dark-field microscope. 2017, 89: 12850–12856
Yang LM, Liu B, Li N, Tang B (2017) Fluorescent nanoprobe for detection and imaging of nucleic acid molecules. Acta Chim Sin 75:1047–1060
Rasheed PA, Sandhyarani N (2017) Electrochemical DNA sensors based on the use of gold nanoparticles: a review on recent developments. Microchim Acta 184:981–1000
Liu QY, Yang YT, Li H, Zhu RR, Shao Q, Yang SG, Xu JJ (2015) NiO nanoparticles modified with 5,10,15,20-tetrakis(4-carboxyl pheyl)-porphyrin: promising peroxidase mimetics for H2O2 and glucose detection. Biosens Bioelectron 64:147–153
Zhang LY, Chen MX, Jiang YL, Chen MM, Ding YN, Liu QY (2017) A facile preparation of montmorillonite-supported copper sulfide nanocomposites and their application in the detection of H2O2. Sensors Actuators B Chem 239:28–35
Sun LF, Ding YY, Jiang YL, Liu QY (2017) Montmorillonite-loaded ceria nanocomposites with superior peroxidase-like activity for rapid colorimetric detection of H2O2. Sensors Actuators B Chem 239:848–856
Ding YN, Yang BC, Liu H, Liu ZX, Zhang X, Zheng XW, Liu QY (2018) FePt-au ternary metallic nanoparticles with the enhanced peroxidase-like activity for ultrafast colorimetric detection of H2O2. Sensors Actuators B Chem 259:775–783
Liu QY, Yang YT, Lv XT, Ding YN, Zhang YZ, Jing JJ, Xu CX (2017) One-step synthesis of uniform nanoparticles of porphyrin functionalized ceria with promising peroxidase mimetics for H2O2 and glucose colorimetric detection. Sensors Actuators B Chem 240:726–734
Liu QY, Chen PP, Xu Z, Chen MM, Ding YN, Yue K, Xu J (2017) A facile strategy to prepare porphyrin functionalized ZnS nanoparticles and their peroxidase-like catalytic activity for colorimetric sensor of hydrogen peroxide and glucose. Sensors Actuators B Chem 251:339–348
Chen MM, Sun LF, Ding YN, Shi ZQ, Liu QY (2017) N,N’-di-caboxymethyl perylene diimides functionalized magnetic nanocomposites with enhanced peroxidase-like activity for colorimetric sensor of H2O2 and glucose. New J Chem 41:5853–5862
Gao FL, Du Y, Yao JW, Zhang YZ, Gao J (2015) A novel electrochemical biosensor for DNA detection based on exonuclease III-assisted target recycling and rolling circle amplification. RSC Adv 5:9123–9129
Gao FL, Lei JP, Ju HX (2013) Ultrasensitive fluorescence detection of bleomycin via exonuclease III-aided DNA recycling amplification. Chem Commun 49:7561–7563
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382:607–609
Jung YK, Park HG (2015) Colorimetric detection of clinical DNA samples using an intercalator- conjugated polydiacetylene sensor. Biosens Bioelectron 72:127–132
Zhang P, Zhang CS, Shu BW (2016) Micropatterned paper devices using amine-terminatedpolydiacetylene vesicles as colorimetric probes for enhanced detection of double-stranded DNA. Sensors Actuators B Chem 236:27–34
Kim MI, Park KS, Park HG (2014) Ultrafast colorimetric detection of nucleic acids based on the inhibition of the oxidase activity of cerium oxide nanoparticles. Chem Comm 50:9577–9580
Yan JW, Tian YG, Tan JH, Huang ZS (2015) Colorimetric and fluorescence detection of G-quadruplex nucleic acids with a coumarin-benzothiazole probe. Analyst 140:7146–7149
Chau LY, He QJ, Qin AL, Yip SP, Lee TMH (2016) Platinum nanoparticles on reduced graphene oxide as peroxidase mimetics for the colorimetric detection of specific DNA sequence. J Mater Chem B 4:4076–4083
Acknowledgements
All authors gratefully acknowledge the financial support of Scientific Research Project of Beijing Educational Committee (Grant No. KM201710028009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 1337 kb)
Rights and permissions
About this article
Cite this article
Liu, Q., Li, L., Zhao, Y. et al. Colorimetric detection of DNA at the nanomolar level based on enzyme-induced gold nanoparticle de-aggregation. Microchim Acta 185, 301 (2018). https://doi.org/10.1007/s00604-018-2833-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2833-3