Abstract
A polydopamine-based molecularly imprinted polymer was deposited on the surface of magnetite (ferroferric oxide) nanoparticles (Fe3O4@PDA MIPs) and is shown to be an efficient and fairly specific sorbent for the extraction of various ochratoxins. The MIPs were characterized by IR spectroscopy and transmission electron microscopy. The adsorption capacities, evaluated through the langmuir adsorption isotherm model, are 1.8, 0.23 and 0.17 mg·g−1 for ochratoxin A, ochratoxin B and ochratoxin C, respectively. Parameters such as the amount of magnetic MIPs, pH value, time for ultrasonication, elution solvent and volume were optimized. Following desorption from the MIP with acetonitrile, the ochratoxins were quantified by HPLC with fluorometric detection. Under optimal experimental conditions, the calibration plots are linear in the range of 0.01–1.0 ng·mL−1 of OTA, 0.02–2.0 ng·mL−1 of OTB, and 0.002–0.2 ng·mL−1 of OTC. The LODs are between 1.8 and 18 pg·mL−1, and the recoveries from spiked samples are 71.0% - 88.5%, with RSDs of 2.3–3.8% in case of rice and wine samples. The MIPs can be re-used for at least 7 times.

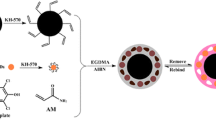
Schematic of the preparation of a magnetic molecularly imprinted polymer based on self-polymerization of dopamine in weakly alkaline solution. Ochratoxins are recognized owing to homologous cavities in the MIPs, and quantified by HPLC after desorption with acetonitrile.


Similar content being viewed by others
References
Kumar R, Ansari KM, Saxena N, Dwivedi PD, Jain SK, Das M (2012) Detection of ochratoxin A in wheat samples in different regions of India. Food Control 26:63–67. https://doi.org/10.1016/j.foodcont.2012.01.004
Zhang YQ, Wang LT, Shen X, Wei XQ, Huang XN, Liu YJ et al (2017) Broad-specificity immunoassay for simultaneous detection of ochratoxins A, B, and C in millet and maize. J Agric Food Chem 65:4830–4838. https://doi.org/10.1021/acs.jafc.7b00770
Wu XM, Hu J, Zhu BH, Lu L, Huang XD, Pang DW (2011) Aptamer-targeted magnetic nanospheres as a solid-phase extraction sorbent for determination of ochratoxin A in food samples. J Chromatogr A 1218:7341–7346. https://doi.org/10.1016/j.chroma.2011.08.045
Xie L, Sheng P, Kong W, Zhao X, Ou-Yang Z, Yang M (2015) Solid-phase extraction using molecularly imprinted polymer for determination of ochratoxin A in human urine. World Mycotoxin J 8:37–44. https://doi.org/10.3920/WMJ2013.1633
Mashhadizadeh MH, Amoli-Diva M, Pourghazi K (2013) Magnetic nanoparticles solid phase extraction for determination of ochratoxin a in cereals using high-performance liquid chromatography with fluorescence detection. J Chromatogr A 1320:17–26. https://doi.org/10.1016/j.chroma.2013.10.062
National Standard of People’s Republic of China (GB 2761-2017), Mycotoxins limited in food, National Health and Family Planning Commission of the People’s Repubic of China, Beijing, 2017. http://mall.foodmate.net/goods-85839.html
Remiro R, González-Peñas E, Lizarraga E, de Cerain AL (2012) Quantification of ochratoxin A and five analogs in Navarra red wines. Food Control 27:139–145. https://doi.org/10.1016/j.foodcont.2012.03.006
Remiro R, Ibáñez-Vea M, González-Peñas E, Lizarraga E (2010) Validation of a liquid chromatography method for the simultaneous quantification of ochratoxin A and its analogues in red wines. J Chromatogr A 1217:8249–8256. https://doi.org/10.1016/j.chroma.2010.11.004
Savastano ML, Losito I, Pati S (2016) Rapid and automatable determination of ochratoxin A in wine based on microextraction by packed sorbent followed by HPLC-FLD. Food Control 68:391–398. https://doi.org/10.1016/j.foodcont.2016.04.016
Rodríguez-Cabo T, Rodríguez I, Ramil M, Cela R (2016) Liquid chromatography quadrupole time-of-flight mass spectrometry selective determination of ochratoxin A in wine. Food Chem 199:401–408. https://doi.org/10.1016/j.foodchem.2015.12.036
Cao JL, Kong WJ, Zhou SJ, Yin LH, Wan L, Yang MH (2013) Molecularly imprinted polymer-based solid phase clean-up for analysis of ochratoxin A in beer, red wine, and grape juice. J Sep Sci 36:1291–1297. https://doi.org/10.1002/jssc.201201055
Maier NM, Buttinger G, Welhartizki S, Gavioli E, Lindner W (2004) Molecularly imprinted polymer-assisted sample clean-up of ochratoxin A from red wine: merits and limitations. J Chromatogr B 804:103–111. https://doi.org/10.1016/j.jchromb.2004.01.014
Giovannoli C, Passini C, Nardo FD, Anfosso L, Baggiani C (2014) Determination of ochratoxin A in Italian red wines by molecularly imprinted solid phase extraction and HPLC analysis. J Agric Food Chem 62:5220–5225. https://doi.org/10.1021/jf5010995
Niu MH, Pham-Huy C, He H (2016) Core-shell nanoparticles coated with molecularly imprinted polymers: a review. Microchim Acta 183:2677–2695. https://doi.org/10.1007/s00604-016-1930-4
Li HF, Xie T, Ye LL, Wang YW, Xie CG (2017) Core-shell magnetic molecularly imprinted polymer nanoparticles for the extraction of triazophos residues from vegetables. Microchim Acta 184:1011–1019. https://doi.org/10.1007/s00604-017-2096-4
Zhang WL, Li Y, Wang Q, Wang C, Wang PF, Mao K (2013) Performance evaluation and application of surface-molecular-imprinted polymer-modified TiO2 nanotubes for the removal of estrogenic chemicals from secondary effluents. Environ Sci Pollut R 20:1431–1440. https://doi.org/10.1007/s11356-012-0983-0
Shi XX, Xu L, Duan HQ, Huang YP, Liu ZS (2011) CEC separation of ofloxacin enantiomers using imprinted microparticles prepared in molecular crowding conditions. Electrophoresis 32:1348–1356. https://doi.org/10.1002/elps.201000515
Turan E, Sahin F (2016) Molecularly imprinted biocompatible magnetic nanoparticles for specific recognition of Ochratoxin A. Sensors Actuators B 227:668–676. https://doi.org/10.1016/j.snb.2015.12.087
Zare F, Ghaedi M, Daneshfar A, Ostovan A (2015) Magnetic molecularly imprinted polymer for the efficient and selective preconcentration of diazinon before its determination by high-performance liquid chromatography. J Sep Sci 38:2797–2803. https://doi.org/10.1002/jssc.201500383
Huang ZZ, Lee HK (2015) Study and comparison of polydopamine and its derived carbondecorated nanoparticles in the magnetic solid-phaseextraction of estrogens. J Chromatogr A 1414:41–50. https://doi.org/10.1016/j.chroma.2015.08.039
Yang B, Lv SF, Chen F, Liu C, Cai CQ, Chen CY et al (2016) A resonance light scattering sensor based on bioinspired molecularlyimprinted polymers for selective detection of papain at trace levels. Anal Chim Acta 912:125–132. https://doi.org/10.1016/j.aca.2016.01.030
Hu MH, Huang PC, Suo LL, Wu FY (2017) Cetylpyridinium chloride functionalized silica-coated magnetite microspheres for the solid-phase extraction and pre-concentration of ochratoxin A from environmental water samples with high-performance liquid chromatographic analysis. J Sep Sci 40:2431–2437. https://doi.org/10.1002/jssc.201601464
Yao GH, Liang RP, Huang CF, Wang Y, Qiu JD (2013) Surface plasmon resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal Chem 85:11944–11951. https://doi.org/10.1021/ac402848x
National Standard of People’s Republic of China (GB 5009.96-2016), Determination of ochratoxin A in food, National Health and Family Planning Commission of the People’s Repubic of China, Beijing, 2016. http://mall.foodmate.net/goods-88895.html
Chen J, Liang RP, Wang XN, Qiu JD (2015) A norepinephrine coated magnetic molecularly imprinted polymer for simultaneous multiple chiral recognition. J Chromatogr A 1409:268–276. https://doi.org/10.1016/j.chroma.2015.07.052
Yu P, Sun QL, Li JF, Tan ZJ, Yan YS, Li CX (2015) Magnetic imprinted nanomicrosphere attached to the surface of bacillus using miniemulsion polymerization for selective recognition of 2,4,6-trichlorophenol from aqueous solutions. J Ind Eng Chem 29:349–358. https://doi.org/10.1016/j.jiec.2015.04.014
Dai SL, Wu SJ, Duan N, Wang ZP (2016) A luminescence resonance energy transfer based aptasensor for the mycotoxin Ochratoxin A using upconversion nanoparticles and gold nanorods. Microchim Acta 183:1909–1916. https://doi.org/10.1007/s00604-016-1820-9
Qing Y, Li X, Chen S, Zhou XP, Luo M, Xu X, Li CR, Qiu JF (2017) Differential pulse voltammetric ochratoxin A assay based on the use of an aptamer and hybridization chain reaction. Microchim Acta 184:863–870. https://doi.org/10.1007/s00604-017-2080-z
Zhang CC, Tang J, Huang LL, Li YP, Tang DP (2017) In-situ amplified voltammetric immunoassay for ochratoxin A by coupling a platinum nanocatalyst based enhancement to a redox cycling process promoted by an enzyme mimic. Microchim Acta 184:2445–2453. https://doi.org/10.1007/s00604-017-2223-2
Wu H, Liu RJ, Kang XJ, Liang CY, Lv L, Guo ZJ (2018) Fluorometric aptamer assay for ochratoxin A based on the use of single walled carbon nanohorns and exonuclease III-aided amplification. Microchim Acta 185:27. https://doi.org/10.1007/s00604-017-52592-6
Jia W, Chu XG, Ling Y, Huang JR, Chang J (2014) Multi-mycotoxin analysis in dairy products by liquid chromatographycoupled to quadrupole orbitrap mass spectrometry. J Chromatogr A 1345:107–114. https://doi.org/10.1016/j.chroma.2014.04.021
Flores-Flores ME, González-Peñas E (2017) An LC–MS/MS method for multi-mycotoxin quantification in cow milk. Food Chem 218:378–385. https://doi.org/10.1016/j.foodchem.2016.09.101
Limay-Rios V, Miller JD, Schaafsma AW (2017) Occurrence of Penicillium verrucosum, ochratoxin A, ochratoxin B and citrinin in onfarm stored winter wheat from the Canadian Great Lakes region. PLoS One 12:e0181239. https://doi.org/10.1371/journal.pone.0181239
Acknowledgements
We greatly acknowledge the National Natural Science Foundation of China (No. 21765014 and 21505067) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 763 kb)
Rights and permissions
About this article
Cite this article
Hu, M., Huang, P., Suo, L. et al. Polydopamine-based molecularly imprinting polymers on magnetic nanoparticles for recognition and enrichment of ochratoxins prior to their determination by HPLC. Microchim Acta 185, 300 (2018). https://doi.org/10.1007/s00604-018-2826-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2826-2